Figure 6.
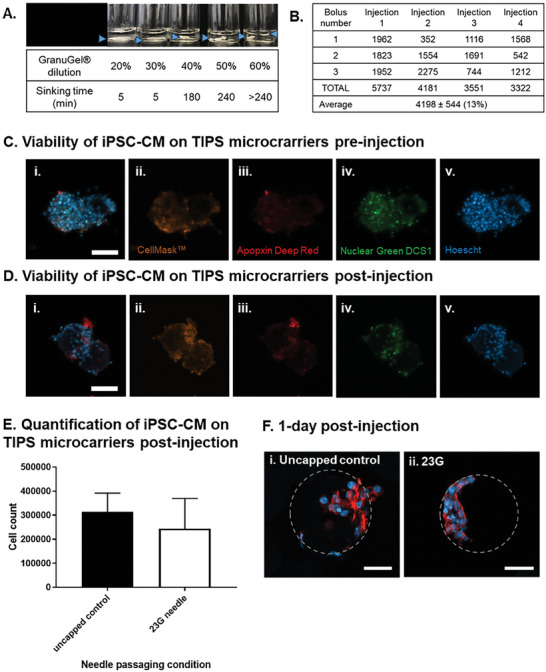
Cellularised TIPS microcarriers are injectable. A) Selection of the GranuGel dilution for delivery of TIPS microcarriers in suspension. Blue arrows indicate the location of the sinking TIPS microcarriers suspension in the tube. 1 × 106 enriched Day 18 iPSC‐CM were seeded on 20 mg of microcarriers and incubated under static dynamic conditions for 24 hours. The media was replaced and the cells were left to recover for another 72 hours. 4 days after cell seeding, the sample was resuspended in 600 µL of 60% GranuGel and injected through either a 23G needle capped or an uncapped syringe into a 24 well low‐bind plate and the samples were immediately analysed or cultured in EB 2% media for 6 days. B) Quantification of the number of TIPS microcarriers delivered in an injection. Each injection consisted of 3 boluses of 50 µL for the total delivery volume of 150 µL. C,D) The viability of iPSC‐CM on TIPS microcarriers was assessed pre‐ and post‐injection (i) Composite image acquired from over imposing (ii) CellMask™ orange plasma membrane stain, (iii) Apopxin deep red, (iv) necrosis marker Nuclear green DCS1 and (v) viability marker Hoescht. E) The mean number of purified iPSC‐CM remaining attached to TIPS microcarriers following delivery through a 23G needle was not different compared with the number of cells remaining attached to microcarriers following delivery through an uncapped syringe. The number of cells in the sample was quantified using a Chemometec automated cell counter. F) 1 day after injection samples were fixed and stained for nuclear (DAPI blue) and cytoskeleton (phalloidin red) markers. Cellularised microcarriers injected through (i) Control = uncapped syringe, (ii) 23G = 23G needle capped syringe. Scale bars represent 50 µm. Microcarriers are outlined in grey. Data are presented as mean ±SD. The significance of the data was calculated by unpaired two‐tailed student's t‐test. (n = 5‐6, p = NS).
