Figure 1.
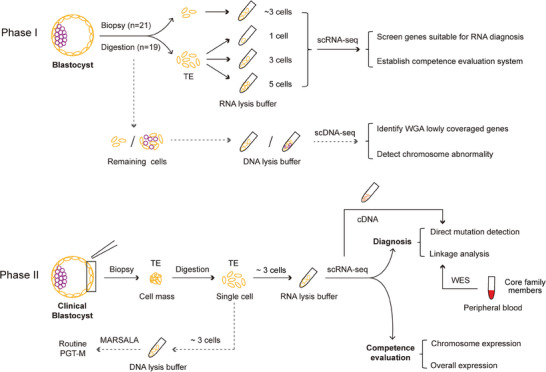
Schematic illustration of this study. (Top) Phase I, feasibility assessment of RNA‐based PGT. Nineteen donated blastocysts were digested, and the TE cells were divided into three groups containing 1, 3, and 5 cells, which were then subjected to RNA‐seq. Twenty‐one blastocysts were biopsied and ≈3 TE cells were collected for subsequent RNA‐seq. In parallel, DNA‐seq experiments were also performed for all blastocysts. The solid lines show the experiment and analysis pipelines based on RNA‐seq data. The dotted lines show the pipeline of DNA‐seq used for the comparison. (Bottom) Phase II, the application strategy for RNA‐based PGT in the clinic. The process comprises three parts: direct mutation detection, linkage analysis, and competence evaluation. The solid lines represent the RNA‐based PGT pipeline, and the dotted lines represent the routine PGT analysis used for diagnostic comparison.
