Figure 5.
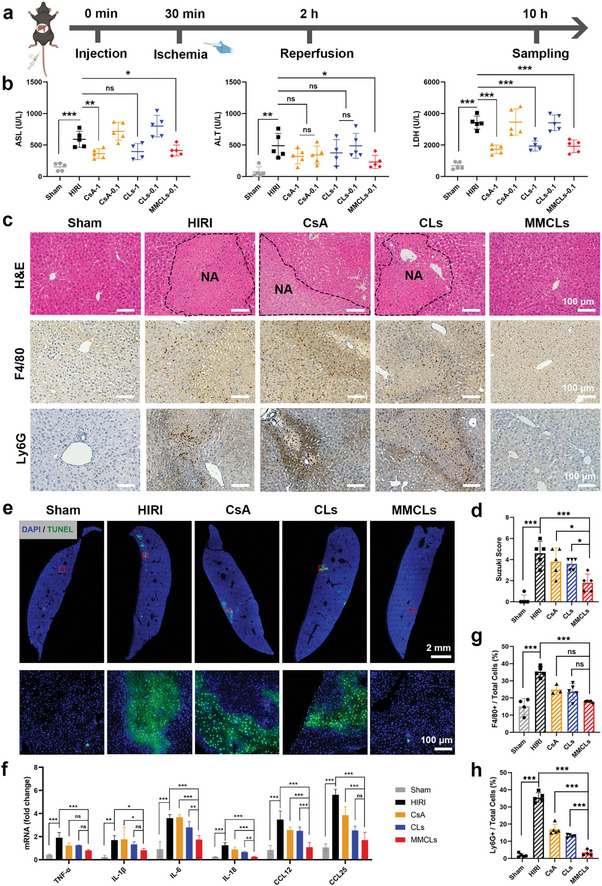
Alleviation of the hepatic injury by CsA, CLs, and MMCLs in a HIRI mouse model. a) Schematic illustration for the drug treatments in HIRI mice. C57BL/6 mice were intravenously injected with PBS, CsA, CLs, or MMCLs, followed by an I/R procedure, and then the blood and liver samples were collected 10 h after injection. A sham‐operated group of healthy mice (Sham) was set as a control. b) Evaluation of liver functions of AST, ALT, and LDH after administration of PBS, CsA, CLs, or MMCLs at a CsA dosage of 0.1 or 1 mg kg−1 (n = 5). c) H&E staining and immunohistochemical staining of liver sections in different treatment groups. NA, necrotic area. Macrophages were stained using F4/80 and neutrophils using Ly6G. d) Suzuki scores of the H&E staining (n = 5). e) TUNEL images visualized by a panoramic scanning microscope and the enlarged images of the red boxes. f) The mRNA expression of inflammatory cytokines and chemokines in hepatic tissues was measured by RT‐qPCR (n = 5). Percentage of g) macrophages (F4/80+) and h) neutrophils (Ly6G+) in total cells of liver sections after the indicated treatments (n = 5). Data are presented as mean ± SD. The statistical significance was analyzed using one‐way ANOVA following Tukey's multiple comparisons test (*p < 0.05; **p < 0.01; ***p < 0.001, ns = no significance).
