Figure 5.
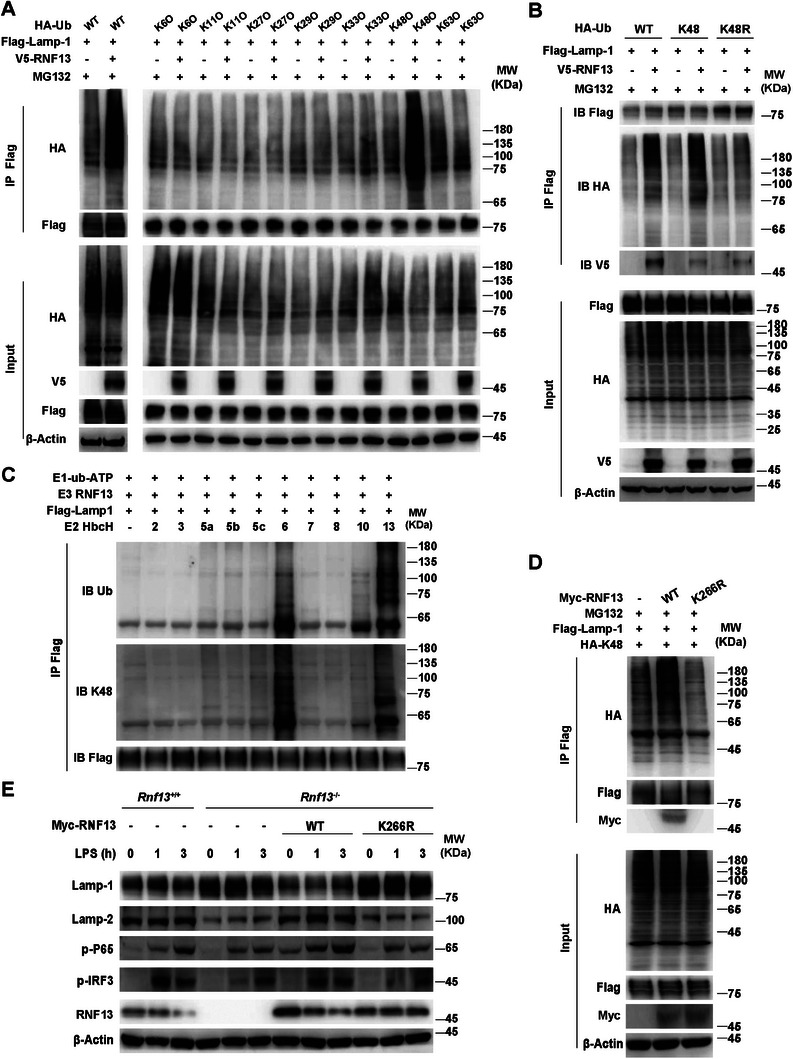
RNF13 induces LAMP‐1 degradation by K48‐linked polyubiquitination. A) Co‐immunoprecipitation analysis of ubiquitin type mediated by RNF13 on LAMP‐1 in HEK293T cells transfected with RNF13, LAMP‐1 and WT or K6/K11/K27/K29/K33/K48/K63 mutants of ubiquitin (Ub), and treated with MG132 (3 µM) for 5 h. B) Co‐immunoprecipitation analysis of ubiquitin type mediated by RNF13 on LAMP‐1 in HEK293T cells transfected with RNF13, LAMP‐1 and WT or K48/K48R of ubiquitin (Ub), and treated with MG132 (3 µM) for 5 h. C) In vitro ubiquitination assay of ubiquitination of LAMP‐1 mediated by RNF13 and related E2 enzymes (HbcH; numbers above lanes indicate that enzyme). D) Co‐immunoprecipitation analysis of K48‐linked polyubiquitination mediated by RNF13 (WT and K266R) on LAMP‐1 in HEK293T cells transfected with RNF13 (WT or K266R), LAMP‐1 and K48 of ubiquitin (Ub), and treated with MG132 (3 µM) for 5 h. E) Western blot assay of RNF13's effect on TLR4 signaling pathway in Rnf13 +/+, Rnf13 −/− RAW264.7 cells and Rnf13 −/− RAW264.7 cells overexpressed by WT or K266R of RNF13 respectively, treated with LPS (100 ng mL−1) for indicated hours. Data are representative of three independent experiments (A–E).
