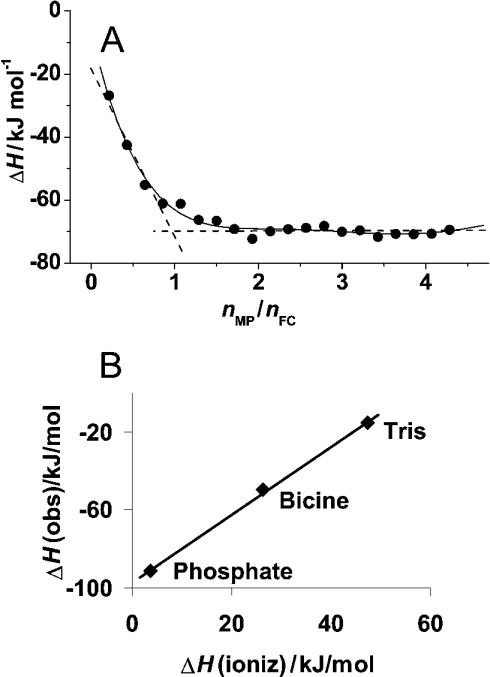
Figure 2. ITC of wild-type ferrochelatase with mesoporphyrin.
(A) Titration in Bicine buffer. Experiments were performed at 25 °C. The plot represents the integral heat for the titration in Bicine buffer of mesoporphyrin (336 μM, 8.6 μl/injection) into ferrochelatase (15 μM) versus the mesoporphyrin to ferrochelatase molar ratios (each point represents an injection). The continuous line is a guide for the eye and the broken lines show the crossing at a 1:1 binding stoichiometry. (B) Effect of buffer on the observed enthalpies. The observed enthalpy changes for the binding reaction of mesoporphirin to ferrochelatase (ΔHobs) in the three studied buffers are plotted as a function of the ionization enthalpy of the respective buffer as reported in [44]. The slope of the fitted curve yields the number of protons released by the buffer and the intercept yields the binding enthalpy (ΔHo) that would be observed in a buffer with ΔHionization=0 [see eqn (1) in the text].