Figure 15.
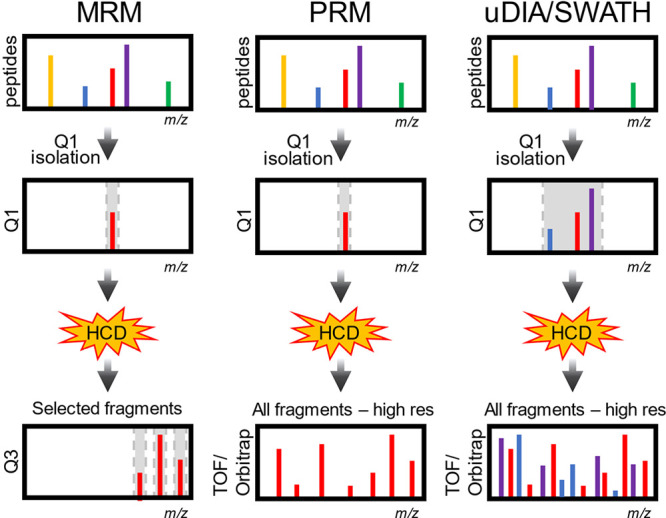
Types of DIA. (A) SRM/MRM. Peptides are ionized by ESI and although there are many peptides entering the mass spectrometer at any time, the first quadrupole (Q1) isolates one mass, which is then fragmented by HCD. Fragment masses from the peptide are then selected in the third quadrupole (Q3). This leads to very low noise and high sensitivity. (B) PRM. Like MRM, peptides are selected in the first quadrupole, but this analysis is done on a high-resolution instrument like an Orbitrap or TOF. Selectivity is gained by exploiting the high mass accuracy and resolution to monitor multiple fragment ions. (C) uDIA/SWATH. Like MRM and PRM, peptides are isolated with Q1, but in this case a much wider isolation window is used. This usually results in co-isolation of many peptides simultaneously. Fragments from many peptides are measured with high resolution and high mass accuracy. Special software is used to get peptide identities and quantities from the fragment ions.
