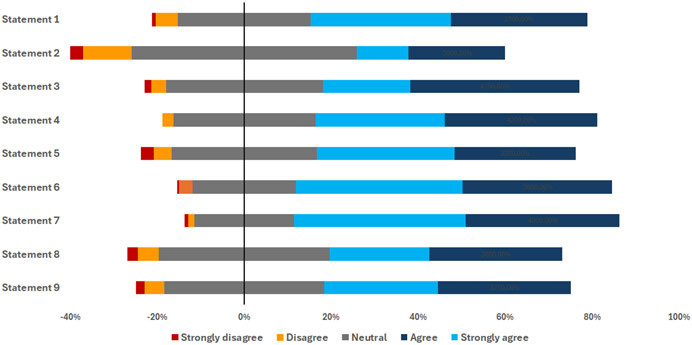
Figure 2.
Participants’ agreement with statements on strategies to manage conflicts of interest. Statement 1: Complete transparency in reporting conflicts of interest is necessary for patients and their advocates; Statement 2: Restricting physician– industry interactions could be one potentially effective option to consider, particularly in light of the evidence that restriction policies may improve prescribing behaviors; Statement 3: Encouraging multiple pharmaceutical companies to collaborate on supporting various access initiatives could mitigate bias favoring a specific product and potentially establish a sustainable framework for these activities; Statement 4: Research on industry-oncologist relationships should be supported and promoted by hospitals and academic institutions; Statement 5 Cancer societies should independently select scientific committees, content during their meetings and speakers should be forbidden from promoting any specific cancer medicine; Statement 6: Medical schools should incorporate programs to increase awareness of potential conflicts of interest with the pharmaceutical industry; Statement 7: Oncology societies and academic institutions should develop and use clear conflict of interest declaration policies for all speakers of their events; Statement 8: Countries should build programs to publicly report payments to doctors from the pharmaceutical industry; Statement 9: A third-party or intermediary entity (such as an oncology society) could collect donations from various pharmaceutical companies and allocate the funds towards supporting research and educational activities, rather than directly providing them to individual oncologists.