Abstract
Ion channel genes have been discovered in many microbial organisms. We have investigated a microbial TRP (transient receptor potential) ion channel gene which has most similarity to polycystic-kidney-disease-related ion channel genes. We have shown that this gene (pkd2) is essential for cellular viability, and is involved in cell growth and cell wall synthesis. Expression of this gene increases following damage to the cell wall. This fission yeast pkd2 gene, orthologues of which are found in all eukaryotic cells, appears to be a key signalling component in the regulation of cell shape and cell wall synthesis in yeast through an interaction with a Rho1-GTPase. A model for the mode of action of this Schizosaccharomyces pombe protein in a Ca2+ signalling pathway is hypothesized.
Keywords: ion channel, PKD2, polycystic kidney disease, polycystin, Schizosaccharomyces pombe, transient receptor potential
Abbreviations: BODIPY, 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene; EMM, Edinburgh minimal medium; GAP, GTPase-activating protein; GEF, guanine nucleotide-exchange factor; GFP, green fluorescent protein; HA, haemagglutinin; LC, long-chain; ME, malt extract; NHS, N-hydroxysuccinimido; ORF, open reading frame; Pkd, polycystic kidney disease; TRP, transient receptor potential; YES, yeast extract medium with supplements
INTRODUCTION
Ion channels are found not only in animals and plants, but also in bacteria, archaea, protists, flagellates and fungi. The investigation of microbial ion channels has led to important discoveries into ion channel structure/function [1] and has resulted in insights into signalling pathways within prokaryotic and eukaryotic cells [1–3]. However, to date, although the number of ion channel genes discovered in microbial genomes has increased, there is little information regarding the role of these genes in microbial cell physiology.
Additionally, microbes and, in particular, yeasts have been used as models to decipher many basic cell processes [4,5]. The molecules involved in cell wall synthesis and cell shape determination have been investigated in fission yeast (Schizosaccharomyces pombe) and have been found to involve a signalling complex containing glucan synthase, a Rho-GTPase, GEF (guanine nucleotide-exchange factors) and GAPs (GTPase-activating proteins) [6–9]. However, the exact signalling event which results in activation of this complex has not yet been described.
In humans, autosomal dominant polycystic kidney disease (Pkd) is one of the most commonly inherited disorders, with an incidence of approx. 1 in 1000 [10]. The disease is characterized by the formation of large fluid-filled cysts in kidneys caused by abnormal differentiation and proliferation of kidney tubular epithelial cells, which result in chronic renal failure in 50% of patients by the age of 60 [11]. Cystic epithelial cells display changes in proliferation, apoptosis, differentiation, polarity, extracellular matrix synthesis and fluid transport [12–15]. In 15% of patients, the causative mutation is located in the pkd2 gene [16,17], which belongs to the family of TRP (transient receptor potential) ion channel genes which are thought to be involved in cellular sensing of temperature, touch, pain, osmolarity, pheromones, taste and other stimuli [18]. Evidence suggests that the polycystin complex (which includes PKD2) may act as a mechanosensor, receiving signals from the extracellular matrix, adjacent cells and tubule lumen (through cilia) and transducing them into cellular responses that regulate proliferation, adhesion, migration, differentiation and maturation essential to the control of the diameter of renal tubules and kidney morphogenesis [19]. In the present study, we have investigated a TRP-like PKD2-related gene in Schiz. pombe which appears to be involved in cell wall synthesis and cell growth through a Rho-GTPase signalling pathway.
EXPERIMENTAL
Schiz. pombe methods
All general methods for Schiz. pombe culture and genetic manipulation were as described previously [20]. Rich medium [YES (yeast extract medium with supplements)], EMM (Edinburgh minimal medium) and malt extract (ME) were as described in [20]. Strains were grown in EMM with appropriate supplements unless stated otherwise [20].
pkd2 gene analysis
Blast searches were performed using the NCBI database (http://www.ncbi.nlm.nih.gov). Alignment and phylogenetic analysis of PKD2-related genes were carried out using ClustalW at http://www.ebi.ac.uk/clustalw [21]. Transmembrane prediction plots were performed using a DAS transmembrane prediction program at http://www.sbc.su.se/~miklos/DAS/ [22].
Schiz. pombe strains used
Strains 96116, h+ his3-D1 leu1-32 ura4-D18 ade6-M210, and 96117, h− his3-D1 leu1-32 ura4-D18 ade6-M216, were obtained from A.T.C.C. (Manassas, VA, U.S.A.)
Plasmids used
pREP41x, pREP42x, pREP41eGFPC and pREP41pkc (v5 epitope) are leucine or uracil Schiz. pombe tagging vectors containing medium-strength thiamin-repressible promoters as described previously [23,24]. pREP3x is a leucine-based vector containing a high-strength thiamin-repressible promoter as described previously [24]. pNR228 is a uracil-based plasmid containing a thymidine kinase gene which confers sensitivity to FuDr as described previously [25]. p81-rho1-HA and gms1-GFP constructs were as described previously [26,27].
Plasmids constructed in the present study
The following plasmids were constructed: pREP41x-pkd2, pREP41x containing the entire pkd2 ORF (open reading frame); pREP41x-pkd2-GFP, C-terminally GFP (green fluorescent protein)-tagged version of pkd2; pREP41x-pkd2-v5, C-terminally v5-epitope-tagged version of pkd2; and pNR228 pkd2 (fragment), pkd2 ORF and 800 bp of upstream and downstream flanking gene sequence.
Disruption of pkd2
Genomic disruption of pkd2 was achieved using the Schiz. pombe his3 gene [28] and the following flanking PCR primers to attach 80 bp of pkd2 flanking sequence to the his3 gene: primer pkd2Flank1, 5′-CTATTCATTAATATCATCTTTTGACTTCACCTTCCTGTTTTTCCATTAATCAACTGTTTCTCACTACCATCAACTGCAAAACCTGCATCTTAGGTTAATT-3′, and primer pkd2Flank2, 5′-ATTGTATAATAATGAAAGAATGTGTAAGGTAGATGGAATGTAATATATATGTAATACGACACAACATTTTCCATCCACGTTTAGCGTAACTCCTTACAAA-3′.
Disruptants were created by lithium acetate transformation as described previously [20]. Primers used to confirm gene disruption identity were (i) pkd2200 bp upstream 5′-TTAATTGGAATTAATAAGTT-3′, (ii) pkd2400 bp reverse 5′-TTAACCGATTACCATACACATAAC-3′, (iii) his3400 bp reverse 5′-ATCCGGATAACGATTGAATTC-3′, (iv) his3 forward 5′-GGAGGTAAGCCTAGTAACGAT-3′ and (v) pkd2200 bp downstream 5′-GATTGCTTATTGAACCTCCTC-3′.
Random spore analysis
Diploid colonies were grown to an A595 of 0.8–0.9 in selective medium at 30 °C. Culture medium (10–15 ml) inoculated 200 ml of ME broth, before growth at 25 °C for 4 days. The culture was checked for the presence of asci under a light microscope. The spores/asci were harvested by centrifugation at 120 g for 5 min, resuspended in 20 ml of 2% glusalase, and incubated overnight at 25 °C. The mixture was plated out on to selective media and incubated at 29 °C for 6 days.
Zymolyase sensitivity assay
Cells were grown in EMM with the appropriate supplements overnight at 30 °C until mid-exponential phase was reached. For the pkd2-overexpressing strains, cells were grown for 14 h in EMM with and without thiamin at 32 °C. Cells were harvested, washed in TE buffer (10 mM Tris/HCl and 1 mM EDTA, pH 7.5), and resuspended at an A600 of 1.0 in the same buffer containing 20 μg of zymolyase-100T per ml. Cell suspensions were incubated at 30 °C, and the A600 was measured at different times.
Cell wall regeneration assay
Cells were washed once in a buffer containing 50 mM sodium citrate and 100 mM sodium phosphate buffer, pH 6.0. The cells were harvested by centrifugation at 225 g for 5 min and incubated with 5 mg/ml NovoZym 234 in the above buffer containing 1.2 M sorbitol. The protoplasts were harvested by centrifugation, then used to inoculate YES medium containing 1.2 M sorbitol, and allowed to regenerate at 30 °C with aeration for 13 h. The frequency of protoplast regeneration was determined by harvesting the regenerating protoplasts at 13 h, diluting them with sterilized water to make them burst and plating them on YES agar. The NovoZym-treated samples were also used to inoculate YES medium and were then plated on YES agar at zero time and 13 h to estimate the number of intact cells present in the samples. The number of colonies formed from intact cells was subtracted from the number of colonies formed from protoplasts to calculate the frequency of regeneration.
β-Glucan level quantification by Aniline Blue dye binding
1,3-β-Glucan levels were quantified with an Aniline Blue dye method. Cells were grown to an A600 of 0.6–0.8, washed twice, and resuspended in TE buffer so that the final A600 was 0.2 for a 0.5 ml cell suspension. NaOH was added to give a final concentration of 1 M, and 1,3-β-glucan was solubilized by incubation in a water bath at 80 °C for 30 min, followed by addition of 2.1 ml of Aniline Blue mixture, consisting of 0.03% (w/v) Aniline Blue, 0.18 M HCl and 0.49 M glycine/NaOH, pH 9.5. The tubes were incubated for 30 min at 50 °C and for an additional 30 min at room temperature (22 °C). Fluorescence was quantified with a spectrofluorimeter, with excitation at 400 nm and emission at 460 nm. For the pkd2-overexpressing strains, cells were cultured in promoter-induced or promoter-repressed conditions for 14 h before assaying.
Confocal microscopy
Confocal microscopy was performed on a Leica 650T instrument using 568 nm and 488 nm filters. Golgi labelling was performed using BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) TR C5-ceramide (Molecular Probes). BODIPY TR C5-ceramide (5 mg) was dissolved in 10 mM Hepes, pH 7.4, to a stock concentration of 0.5 mM. Mid-exponential phase cells were spun and washed in ice-cold 10 mM Hepes, pH 7.4, and BODIPY TR C5-ceramide was added at a working concentration of 50 μM. The cells were incubated at 4 °C for 30 min and subsequently washed four times in ice-cold 10 mM Hepes, pH 7.4. The cells were finally resuspended in YES medium, and incubated at 30 °C for 30 min with agitation before mounting for confocal microscopy.
Surface biotinylation
Surface biotinylation of Schiz. pombe cells co-expressing pkd2-v5 and gms1-GFP was performed as described previously for Candida albicans [29]. Cells were broken before or after biotinylation using glass beads and vortex-mixing for 5 min at 4 °C. Cell lysis was viewed microscopically and was determined to be approx. 60%. For biotinylation of cells after three washing steps in cold PBS, pH 7.4, cells were incubated for 2 h at 4 °C with 10 mg/ml sulpho-NHS-LC-biotin (NHS is N-hydroxysuccinimido and LC is long-chain) (Molecular Probes) in binding buffer, 50 mM NaHCO3, pH 8.5. The remaining reactive sulpho-NHS-LC-biotin was blocked by adding 2 vol. of 100 mM Tris/HCl, pH 8.0, and further incubation for 1 h. Cells were harvested by centrifugation at 300 g for 20 min at 4 °C and washed twice in cold PBS pH 7.4 and once in PBS, pH 7.4, containing 1 mM EDTA, 1% (v/v) Triton X-100, 150 mM NaCl and 1 mM dithiothreitol, with protease inhibitors. Cell debris was removed by centrifugation at 300 g for 20 min at 4 °C. Biotinylated proteins were purified using Immunopure-immobilized avidin (Pierce), according to the manufacturer's instructions. Samples were analysed by SDS/PAGE and immunoblotting with anti-v5 or anti-GFP antibodies (Invitrogen), and subsequently with an anti-mouse or anti-rabbit horseradish-peroxidase-conjugated secondary antibody, and developed with a Supersignal chemiluminescent detection kit (Pierce).
Immunoprecipitation
Spheroplast lysates were prepared as described below. Membrane fractions were isolated by centrifugation at 100000 g for 2 h at 4 °C. The pellet fraction was resuspended in 0.6 ml of buffer A (1 mM EDTA, 1% Triton X-100, 150 mM NaCl, 1 mM dithiothreitol, and protease inhibitors used for spheroplasting, in PBS, pH 7.4) and incubated on ice for 30 min. A 2 μg amount of the appropriate antibody against the v5 epitope or HA (haemagglutinin) tag was added overnight at 4 °C on a rotating wheel. The immunoprecipitation was performed using a Classic A Immunoprecipitation kit (Pierce), according to the manufacturer's instructions. Following elution, the samples were mixed with SDS sample buffer (Sigma). Samples were analysed by SDS/PAGE and immunoblotting with anti-v5 or anti-HA antibodies, and subsequently with an anti-mouse or anti-rabbit horseradish-peroxidase-congugated secondary antibody and developed with a Supersignal chemiluminescent detection kit.
Solubilization of pkd2 by Triton X-100
Spheroplasts were prepared from cells and lysed as described below. Membrane fractions were isolated by centrifugation at 100000 g. The supernatant that contained soluble proteins were precipitated in 10% trichloroacetic acid and washed with acetone before SDS/PAGE separation. Membrane fractions (pellet) were untreated or treated with 0.2 M Na2CO3 (pH 11) or 1% Triton X-100 for 30 min on ice and re-fractionated at 100000 g for 2 h at 4 °C.
Preparation of spheroplast lysates
Five A600 units of Schiz. pombe cells were converted into spheroplasts with 0.8 mg of zymolyase-20T and 80 units of glusalase per ml of spheroplasting buffer (50 mM Tris/HCl, pH 7.4, 1 M sorbitol, 1 mM dithiothreitol and 1 mM 2-mercaptoethanol). Spheroplasts were resuspended in 0.6 ml of HEGN100 buffer (20 mM Hepes, pH 7.9, 1 mM EDTA, 10% glycerol and 100 mM NaCl) supplemented with 1 mM PMSF, 8 μg/ml aprotinin, 4 μg/ml pepstatin and 2 μg/ml leupeptin. Spheroplasts were lysed by three rounds of freezing in a liquid-nitrogen bath and rapid thawing at 30 °C.
Expression analysis of pkd2 following cell wall damage
Cells containing a genomic copy of pkd2 with a C-terminal v5 epitope tag were grown to an A600 of 0.6. Cells were harvested, washed in TE buffer, and resuspended at an A600 of 1.0 in the same buffer containing 20 μg of zymolyase-100T per ml. Cell suspensions were incubated at 30 °C for various times (0, 1 and 2 h). Following cell wall damage, the cells were harvested by centrifugation at 300 g for 20 min at 4 °C and washed twice in PBS, followed by resuspension in PBS, pH 7.4, containing 1 mM EDTA, 1% Triton X-100, 150 mM NaCl and 1 mM dithiothreitol, with protease inhibitors. The cells were broken using glass beads and vortex-mixing for 5 min at 4 °C. Cell lysis was viewed microscopically and was determined to be approx. 60%. Cell debris was removed by centrifugation at 300 g for 20 min at 4 °C. The lysates were mixed with SDS sample buffer. Samples were analysed by SDS/PAGE and immunoblotting with anti-v5 antibodies, and subsequently with an anti-mouse secondary antibody and developed with a Supersignal chemiluminescent detection kit.
RESULTS
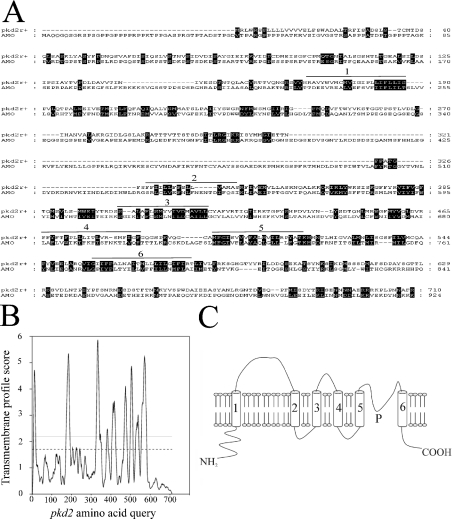
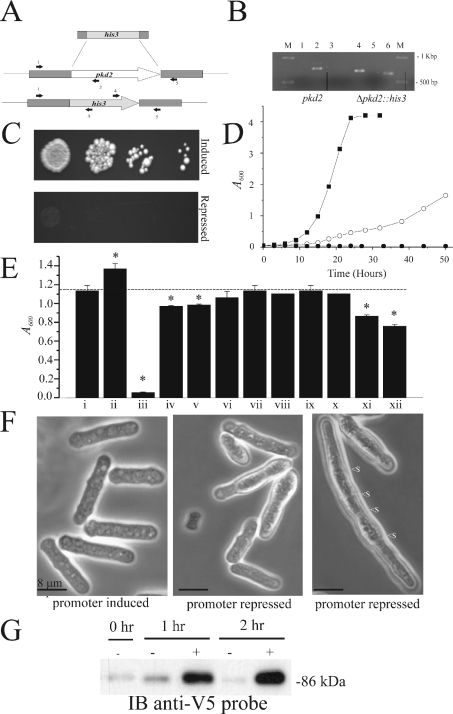
PKD2-related genes have been described in the model organisms Caenorhabditis elegans, Drosophila melanogaster and Danio rerio [30–32]. In the present paper, we report on a PKD2-related gene in Schiz. pombe. The gene was discovered by BLAST analysis using the amino acid sequence of the sixth transmembrane domain of the yeast vacuolar channel, which is a TRP ion channel gene from Saccharomyces cerevisiae [33], as a probe against the Schiz. pombe genomic sequence. Of several TRP channel orthologues found, one (SPAC1F7.03, now called pkd2) possesses significant amino acid similarity (46% in the six predicted transmembrane domains) to a PKD2-related gene called AMO (almost there) in D. melanogaster [31] (Figure 1A). As with other yeast ion channels that have higher eukaryotic orthologues, the most similarity (59%) is observed in the fifth and sixth transmembrane domains [34,35]. Transmembrane analysis plots suggested that the pkd2 gene product in Schiz. pombe possesses six transmembrane domains, which are in agreement with the predictions for AMO and human PKD2 [19,31] (Figure 1B). The predicted N-terminus amino acid sequence of pkd2 contains a signal sequence with a putative cleavage site at 23 amino acids. The amino acid sequence of pkd2 also contains a large loop between the first and second predicted transmembrane domains, which is similar to the structure reported for other PKD2-related genes [30,31] (see the model in Figure 1C). pkd2 appears to be one member of a family of PKD2-related genes in yeast and fungi (results not shown). This family includes (i) the Ca2+-related spray protein from Neurospora crassa, a mutation in which resulted in a spray-type growth pattern and altered hyphal tip Ca2+ [36] and (ii) the S. cerevisiae gene YAL053w, knockout of which resulted in an increased sensitivity to zymolyase (β-glucan-digesting enzyme) (C. P. Palmer, unpublished work). In order to elucidate the function of pkd2 in the cellular physiology of Schiz. pombe, we constructed a knockout cassette by PCR using the Schiz. pombe his3 gene and 80 bp of pkd2 flanking sequence (Figure 2A). The cassette was used to create a diploid strain pkd2/Δpkd2::his3, which was subsequently sporulated, and the resulting spores were investigated by random spore analysis (Table 1). The results indicated that pkd2 is an essential gene and that a haploid pkd2 genomic knockout strain is possible only when pkd2 is provided on a complementing plasmid (under the control of either its own promoter or an inducible promoter). Attempts to remove this complementing pkd2 plasmid (by forced 5′-fluoro-2′-deoxyuridine toxicity with a thymidine kinase gene on the pNR228 construct) resulted in non-viable cells (Table 1). The haploid Δpkd2::his3 strain with complementing pREP41x-pkd2 plasmid was subjected to extensive PCR analysis to confirm the true identity of this knockout (Figure 2B). Various PCR primers were used to confirm the complete removal of the pkd2 gene from the genome (Figure 2A). Additionally, the entire ORF plus 400 bp of upstream and downstream sequence was PCR-amplified and sequenced from this pkd2 genome-deleted strain to confirm the correct insertion of the his3 gene and deletion of the pkd2 gene. In a haploid Δpkd2::his3 strain with a complementing pREP41x-pkd2 (thiamin-repressible promoter) plasmid, cell growth and morphology appeared identical with the wild-type pkd2 strain in the absence of thiamin (Figures 2D and 2E). In contrast, repression of this plasmid's promoter by the addition of thiamin to the medium resulted in non-viable cells on solid medium (Figure 2C). In liquid medium, cells were found to still be viable, although growth rates were dramatically reduced (Figure 2D), stationary phase being reached after 74 hours, indicating that pkd2 may be involved in the control of cell proliferation. Addition of Ca2+ to the medium resulted in reduction of the growth rate to zero (Figure 2D), indicating that pkd2 may be involved in Ca2+ signalling as for other TRP channels. Proliferation of cells without pkd2 repression in the presence of Ca2+ was not significantly altered compared with proliferation in the absence of Ca2+ (results not shown). Significant sensitivity was also found upon pkd2 depletion to the following chemicals: benomyl (a microtubule inhibitor) and amiodarone (a disrupter of Ca2+ homoeostasis in yeast; [37]) (Figure 2E). Addition of 50 mM NaCl or MgCl2 resulted in a small, but significant, decrease in cell growth (Figure 2E). Other agents, such as latrunculin (actin inhibitor) or butanedione monoxime (myosin ATPase inhibitor), had no effect. Similarly, addition of cyclosporin to these cells did not affect cell growth (Figure 2E). Cell growth was marginally improved by the addition of 1 M sorbitol to the medium (Figure 2E), suggesting that the effect of depletion of pkd2 on cell growth is not entirely due to a defect in cell wall formation. For all chemicals, the wild-type strain was not affected at the concentrations tested. The cells in liquid culture 24 h after pkd2 depletion appeared elongated (Figure 2F); average cell length was increased by 31% (compared with the control cells), with 11% of cells containing more than three septa compared with less than 1% for control cells. Additionally, 22% of the cells appeared bulbous and possessed an uneven cell periphery, while 5% of the cells appeared to be dead, which was confirmed by Trypan Blue staining. Treatment of cells with a low concentration of zymolyase resulted in a significant increase in expression of pkd2 (Figure 2G). This suggests that pkd2 may be involved in a signalling response to cell wall damage.
Figure 1. A TRP-like polycystic-kidney-disease-related gene in fission yeast.
(A) The predicted amino acid sequence of the protein encoded by Schiz. pombe pkd2 was compared by ClustalW analysis with the predicted sequence of the D. melanogaster PKD2-related gene (AMO). Predicted transmembrane domains are numbered (1–6). Amino acid similarities between AMO and pkd2 are highlighted. (B) Predicted transmembrane plot of protein encoded by pkd2. Broken line and grey line indicates loose and strict cut-off values for TM prediction respectively. (C) A model of the predicted protein encoded by pkd2; six TM domains (1–6) and a putative pore region (P) are labelled.
Figure 2. pkd2 is an essential gene with a Ca2+-sensitive gene-depletion phenotype.
(A) The knockout cassette used to replace the pkd2 gene. Primers used to confirm the identity of the pkd2-knockout are labelled as arrows (1–5), with their approximate position in the wild-type or knockout genome shown. (B) PCR analysis of the haploid pkd2 genome-deletion strain (pkd2::his3 and pREP41x-pkd2) and the wild-type strain (pkd2) with the following primers as shown in (A). Lanes 1 and 4 (primer set 1 and 3); lanes 2 and 5 (primer set 1 and 2); lanes 3 and 6 (primer set 4 and 5). (C) A haploid pkd2::his3 strain was complemented by a plasmid-borne (pREP41x) pkd2 gene under control of a repressible promoter. Depletion of pkd2 by promoter repression on solid medium. (D) In liquid medium, pkd2-depleted cells were still viable; growth curves are depicted for cells in promoter-induced conditions (■), and promoter-repressed conditions in normal growth medium (○) and in medium with 50 mM Ca2+ (●). (E) Sensitivity of cells with depleted pkd2 to various substances: i, null; ii, 1 M sorbitol; iii, 50 mM Ca2+; iv, 50 mM Na+; v, 50 mM Mg2+; vi, 50 mM K+; vii, low Ca2+; viii, 0.2 μM latrunculin; ix, 2 mM butanedione monoxime; x, 1 μM cyclosporin; xi, 5 μg/ml benomyl; xii, 1 μM amiodarone. Wild-type cells showed no sensitivity to these substances at the concentration stated (results not shown). Asterisks (*) indicate significant values. (F) Confocal microscopy of cells upon depletion of pkd2 by promoter repression of a plasmid-borne copy. Displayed is an elongated cell which contains multiple septa (<s). (G) Analysis of pkd2 expression in cells following treatment with zymolyase. Exponential phase cells containing a genomic copy of pkd2 with a v5 tag were treated with (+) and without (−) zymolyase at the times specified. Cells were subsequently lysed, and the protein extracts were analysed by SDS/PAGE and Western blotting with an anti-v5 antibody. The size of marker proteins in kDa is indicated to the side of the blot.
Table 1. pkd2 is an essential gene.
Random spore analysis of diploid pkd2/Δpkd2::his3 strains. The diploid strain pkd2/Δpkd2::his3 containing the plasmids indicated were sporulated and plated on to minimal medium lacking the indicated nutritional supplements (−Ura, −Ura/−His) to determine the genotype of the resultant haploid progeny. Colonies which appeared on −Ura/−His medium were streaked on to medium containing 5′-fluoro-2′-deoxyuridine (FuDR), which renders cells expressing the thymidine kinase gene (from pNR228) sensitive to this drug. N/A, not applicable.
| Medium | |||
|---|---|---|---|
| Diploid strain constructed and sporulated | −Ura | −Ura/−His | −His/+FuDR |
| pkd2/Δpkd2::his3 and pREP42x-pkd2 | 106 | 46 | + |
| pkd2/Δpkd2::his3 and pREP42x (empty plasmid) | 98 | 0 | N/A |
| pkd2/Δpkd2::his3 and pNR228-pkd2 (3.7 kb genome fragment) | 49 | 22 | − |
| pkd2/Δpkd2::his3 and pNR228 (empty plasmid) | 62 | 0 | N/A |
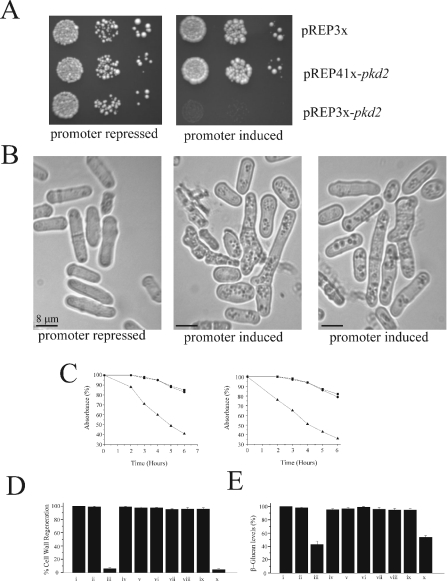
Overexpression of pkd2 resulted in cell death on liquid (results not shown) and solid medium (Figure 3A). Microscopic examination of these cells at 16 h following promoter induction suggested cell lysis, with many bent and kinked cells. Significant changes in cellular morphology were observed: approx. 12% of cells had an altered growth polarization, appearing as small buds growing parallel or at 45° to the long axis of cell growth. Average cell length was increased by 8%, but multiple septa were not observed. This growth defect was not rescued by the addition of sorbitol to either solid or liquid medium.
Figure 3. pkd2 overexpression and depletion cause changes in cellular morphology and alter cell wall formation.
(A) Growth of cells containing a plasmid-borne copy of pkd2 under the control of a high-strength inducible promoter (pREP3x) and a medium-strength inducible promoter (pREP41x) on solid medium after 4 days of growth. (B) Appearance of cells overexpressing pkd2 from pREP3x (promoter-induced and -repressed conditions) under bright-field microscopy. (C) Left-hand panel, zymolyase-sensitivity assay for pkd2-depleted cells; (▲) Δpkd2 and pREP41x-pkd2 (promoter-repressed); (■) Δpkd2 and pREP41x-pkd2 (promoter-induced); (●) pkd2 wild-type strain. Right-hand panel, zymolyase-sensitivity assay for pkd2-overexpressed cells; (▲) pkd2 and pREP3X-pkd2 (promoter-induced); (■) pkd2 and pREP3X-pkd2 (promoter-repressed); (●) pkd2 wild-type strain. (D) Cell wall regeneration assay for various strains containing indicated plasmids: i, wild-type pkd2; ii, Δpkd2 and pREP41x-pkd2 (promoter-induced); iii, Δpkd2 and pREP41x-pkd2 (promoter-repressed); iv, pkd2 and pREP3x (promoter-repressed); v, pkd2 and pREP3x (promoter-induced); vi, Δpkd2 and pNR228-pkd2 (fragment); vii, pkd2 and pREP41x-pkd2 (promoter-repressed); viii, pkd2 and pREP41x-pkd2 (promoter-induced); ix, pkd2 and pREP3X-pkd2 (promoter-repressed); x, pkd2 and pREP3x-pkd2 (promoter-induced). (E) Cell wall β-glucan levels as measured by Aniline Blue dye binding for strains with plasmids and conditions as in (D).
Both the pkd2-depleted and the pkd2-overexpressing strains exhibited significant sensitivity to zymolyase (Figure 3B) (a cell wall β-glucan-digesting enzyme) compared with control cells. This suggested that alteration of pkd2 levels may result in reduced levels of β-glucan in the cell wall, which consists mainly of β-glucan synthesized by a plasma membrane glucan synthase [6]. Indeed, pkd2 depletion or overexpression drastically reduced the ability of the cell to re-synthesize its cell wall, as analysed by cell wall regeneration assays (Figure 3C), suggesting a possible reduction in glucan synthase activity. This was confirmed by measuring the amount of β-glucan in the cell walls of pkd2-depleted and pkd2-overexpressing strains by Aniline Blue dye binding (Figure 3D).
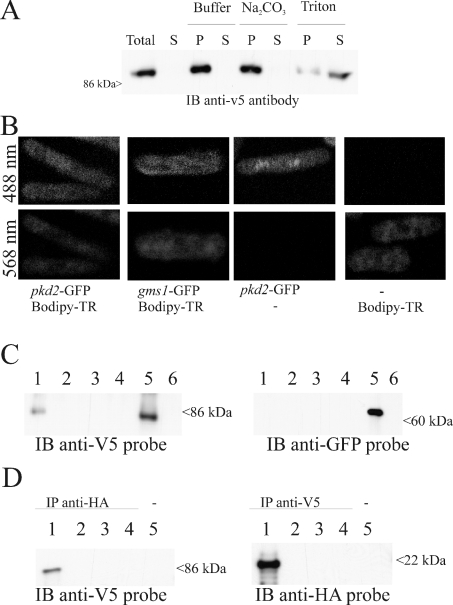
In order to determine the subcellular localization of protein, we constructed GFP and v5 epitope C-terminus-tagged versions of pkd2. These were found to still be functional by plasmid-swapping experiments using a Δpkd2::his3 strain with a complementing pNR228-pkd2 (fragment) construct (results not shown). Furthermore, these tagged versions behaved identically with the untagged expressed gene and the wild-type cells in terms of growth and appearance (results not shown). Cells expressing a pkd2-v5 construct were lysed under different buffer conditions. The PKD2 protein could only be solubilized in a buffer containing Triton X-100, consistent with the predicted transmembrane nature of the protein (Figure 4A). Cells expressing GFP-tagged pkd2 plasmids in Schiz. pombe (Figure 4B) were visualized by confocal microscopy. A weak and punctuate fluorescence was observed within the cytoplasm of the cells, suggesting a possible Golgi localization. A similar pattern of fluorescence was observed for a genomic GFP-tagged pkd2 strain (results not shown). This was confirmed by dual labelling with the Golgi marker BODIPY TR C5-ceramide. Furthermore, expression of a GFP-tagged gms1 gene (a known Golgi-resident UDP-galactose transporter [27]), revealed a similar pattern of fluorescence. No significant fluorescence was observed in endoplasmic reticulum or plasma membranes for cells expressing a GFP-tagged pkd2 construct or genomic GFP-tagged pkd2. Since PKD2 channels have been reported previously to be localized to the Golgi and plasma membrane when endogenously expressed [38], we performed surface biotinylation experiments on cells expressing v5-tagged pkd2. As a control, the Golgi-resident gms1 gene with a GFP tag was also co-expressed. These experiments showed that a minor fraction of the pkd2-GFP protein was present in the plasma membrane (Figure 4C).
Figure 4. pkd2 is localized to the Golgi and plasma membrane and is in a complex with a Rho-GTPase.
(A) Western blot of protein extracted from a Δpkd2 and pREP41x-pkd2-v5 tag (promoter induced) strain solubilized with the indicated reagents. Equivalent amounts of total lysate (Total) and supernatant (S) or pellet (P) fractions were loaded. Pellet fractions obtained from cell lysates were either untreated or incubated in the presence of 0.1 M Na2CO3 at pH 11 and 1% Triton X-100, and centrifuged at 100000 g for 30 min before analysis by immunoblotting (IB). (B) Confocal microscopy of GFP-tagged pkd2. Fluorescent images were recorded at the indicated wavelengths. (C) Surface biotinylation of PKD2 protein. Cells co-expressing pkd2-v5 and gms1-GFP (lanes 1–3, 5 and 6) or containing empty vectors (lane 4) were subjected to biotin labelling (lanes 1 and 3–5) or mock-labelled (lanes 2 and 6). Biotin labelling was performed either before (lanes 1, 3 and 4) or after cell breakage (lane 5). The biotin complexes were purified using streptavidin-coated beads (lanes 1, 2 and 4–6) or non-coated beads (lane 3), and subsequently eluted. The eluates were separated on duplicate SDS/12% PAGE gels and Western blotted. The blots were probed with either an anti-v5 antibody or an anti-GFP antibody. (D) Western blot depicting pkd2 interaction with rho1. Extracts were prepared from cells expressing the following tagged constructs and used for immunoprecipitation. The immunoprecipitates were loaded and separated on SDS/10% PAGE and then analysed by immunoblotting. Lanes 1 and 5, pkd2-v5 and rho1-HA; lane 2, pkd2-v5; lane 3, rho1-HA; lane 4, null. The immunoprecipitating (IP) antibody is shown above each blot, and the antibody used as a probe is indicated below each blot. Size of marker proteins in kDa is indicated to the side of all the blots.
We hypothesized that pkd2 may form part of a cell wall synthesis signalling pathway. Importantly, a v5-epitope-tagged pkd2 immunoprecipitated a HA-tagged rho1 and vice versa (Figure 4D). Rho1 is a small GTP-binding protein which acts as an activating subunit for the plasma membrane β-glucan synthase at the growing tip of the cell [7].
DISCUSSION
We propose that the protein encoded by the pkd2 gene is a key component of a cell wall synthesis signalling pathway. Figure 5 depicts a possible mode of action for the pkd2 gene product in such a pathway. Glucan synthase, which is present in the plasma membrane at the growing tip of the cell, synthesizes β-(1,3)-glucan, the major component of the cell wall [6]. Glucan synthase is activated by rho1 [7]. The predominant localization of pkd2 is within the Golgi membrane; however, a small amount of PKD2 protein is targeted to the plasma membrane, in a immunoprecipitable complex with Rho1. Activation of pkd2 in the plasma membrane may result in Ca2+ entry to the cell, since TRP channels are known to be involved in Ca2+ signalling [18] and pkd2 depletion causes a Ca2+-sensitive phenotype (Figure 2E). Rho GTPases are binary switches, cycling between an inactive GDP-bound form and an active GTP-bound form in the membrane, and transduce signals into the cytoplasm via effector pathways that regulate cell growth, differentiation and apoptosis. In turn, Rho activation is enhanced by GEFs and their deactivation is accelerated by GAPs [39,40]. In Schiz. pombe, several GAPs and GEFs involved in cell wall synthesis and cell morphology have been reported to regulate and/or interact with rho1 [8,9]. Since some GEFs and GAPs have been demonstrated to be modulated by Ca2+ [39,40], and pkd2 is in a complex with rho1, it is conceivable that Ca2+ entry through activation of pkd2 alters a GAP/GEF switch which results in rho1 activation and subsequently glucan synthase activation. Alternatively, rho1 activation of glucan synthase is dependent on prenylation of rho1 by geranylgeranyl transferase I [41]. An S. cerevisiae strain with a mutation in the geranylgeranyl transferase (resulting in decreased rho1 modification and 1,3-β-glucan synthase activity) was restored by addition of Ca2+ [42], suggesting that cytoplasmic Ca2+ may play a role in the regulation of this process. An important question is the nature of the mechanism of activation of pkd2. Within the Schiz. pombe plasma membrane, only two ion channels have been reported: one is a nonselective cation channel and the other a mechanosensitive ion channel [43]. Since polycystin has been implicated in mechanosensation in cilia [44], it is possible that pkd2 encodes a mechanosensitive ion channel. Another question concerns the significance of the subcellular localization of pkd2. As with human PKD2 [19], the fission yeast PKD2 protein is localized mainly to Golgi and, to a lesser extent, to plasma membrane when endogenously expressed. However, pkd2 interacts with rho1, which is localized in a complex with glucan synthase at the growing tip of the cell [7]. It is possible that pkd2 is translocated from the Golgi membrane into the plasma membrane at the growing tip during periods of cell proliferation or following cell wall damage. Indeed, in Madin–Darby canine kidney cells PKD2 subcellular distribution is altered in response to wound healing stress [45]. Since pkd2 expression increases following cell wall damage, it is conceivable that pkd2 is involved in a response to cell wall damage to allow cell wall remodelling. Similarly, expression of mechanosensitive ion channels in bacterial cell membranes has been found to be regulated by the stress σ factor, RpoS, with the number of channels increasing in stationary growth phase when the cell wall undergoes remodelling [46].
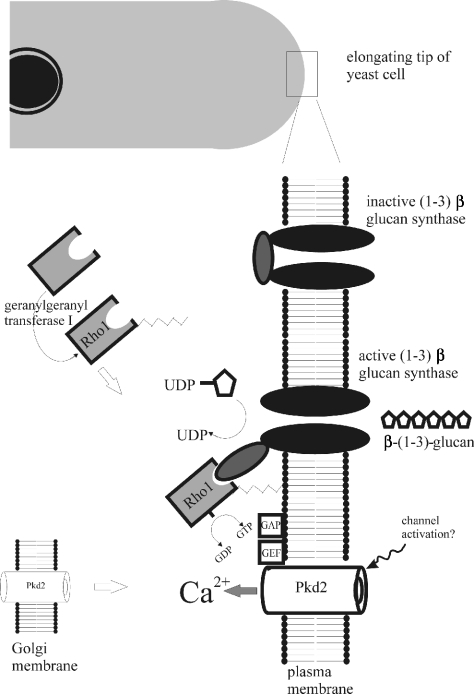
Figure 5. Hypothetical model of a possible pkd2 signalling pathway in fission yeast.
pkd2 is localized to the Golgi membrane and also to the plasma membrane in a complex with rho1, which is present at the elongating cell tip in a complex with glucan synthase. Activation of pkd2 possibly results in Ca2+ entry. Upon Ca2+ entry, rho1 may be activated by the action of a GEF/GAP switch that has been shown to modulate/interact with rho1. Additionally, attachment of rho1 to the plasma membrane requires the activity of a geranylgeranyl transferase which has been suggested to be Ca2+-regulated. Modulation of rho1 affects the glucan synthase enzyme which is responsible for β-glucan synthesis in the cell wall. Alteration of glucan synthase activity causes changes in cell growth, shape and polarization of cell wall synthesis.
In summary, we have shown that the pkd2 gene in Schiz. pombe has significant molecular and structural similarities to higher eukaryotic PKD2s. The gene is essential, and plays a critical role in cell proliferation, cell viability, cell shape and extracellular- matrix synthesis, and functions in a complex with rho1. In conclusion, a PKD2-related gene model in Schiz. pombe could be useful in elucidating the molecular mechanisms of PKD2-related ion channels. Indeed, yeasts have been found to be very tractable models for the study of human genetic disorders [47]. The strengths of using Schiz. pombe as a model organism [48], such as the ease of performing forward and reverse genetics, its short generation time, small size and the ease with which transgenic cells can be generated, have made this organism invaluable in the investigation of many basic cellular mechanisms [4,5]. Furthermore, the power of microbial genetics and the success of obtaining crystallographic structures of microbial ion channels make the study of microbial ion channels a potentially fruitful area for dissecting ion channel structure and function [49]. We anticipate that this pkd2 model in Schiz. pombe could be very useful in deciphering the nature and function of PKD2-related ion channels and their role in the regulation of cell shape and cell size.
Acknowledgments
We thank Janet Leatherwood for providing pNR228, Iain Hagan for pREP41eGFP/pREP41pkc, Issei Marabushi for pREP81 Rho1-HA and Kaoru Takegawa for GMS1p-GFP. This work is funded by a Wellcome Trust project grant to C. P. P.
References
- 1.Kung C., Blount P. Channels in microbes: so many holes to fill. Mol. Microbiol. 2004;53:373–380. doi: 10.1111/j.1365-2958.2004.04180.x. [DOI] [PubMed] [Google Scholar]
- 2.Denis V., Cyert M. S. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 2002;156:29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell Sci. 2004;117:2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- 4.Verde F. On growth and form: control of cell morphogenesis in fission yeast. Curr. Opin. Microbiol. 1998;1:712–718. doi: 10.1016/s1369-5274(98)80120-3. [DOI] [PubMed] [Google Scholar]
- 5.Rupes I. Checking cell size in yeast. Trends Genet. 2002;18:479–485. doi: 10.1016/s0168-9525(02)02745-2. [DOI] [PubMed] [Google Scholar]
- 6.Cortes J. C., Ishiguro J., Duran J. A., Ribas J. C. Localization of the (1,3)β-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell. Sci. 2002;115:4081–4096. doi: 10.1242/jcs.00085. [DOI] [PubMed] [Google Scholar]
- 7.Qadota H., Python C. P., Inoue S. B., Arisawa M., Anraku Y., Zheng Y., Watanabe T., Levin D. E., Ohya Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-β-glucan synthase. Science. 1996;272:279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- 8.Calonge T. M., Arellano M., Coll P. M., Perez P. Rga5p is a specific Rho1p GTPase-activating protein that regulates cell integrity Schizosaccharomyces pombe. Mol. Microbiol. 2003;47:507–518. doi: 10.1046/j.1365-2958.2003.03312.x. [DOI] [PubMed] [Google Scholar]
- 9.Iwaki N., Karatsu K., Miyamoto M. Role of guanine nucleotide exchange factors for Rho family GTPases in the regulation of cell morphology and actin cytoskeleton in fission yeast. Biochem. Biophys. Res. Commun. 2003;312:414–420. doi: 10.1016/j.bbrc.2003.10.140. [DOI] [PubMed] [Google Scholar]
- 10.Gabow P. A. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1993;329:332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 11.Hateboer N., v Dijk M. A., Bogdanova N., Coto E., Saggar-Malik A. K., San Millan J. L., Torra R., Breuning M., Ravine D. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1–PKD2 Study Group. Lancet. 1999;353:103–107. doi: 10.1016/s0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 12.Woo D. Apoptosis and loss of renal tissue in polycystic kidney diseases. N. Engl. J. Med. 1995;333:18–25. doi: 10.1056/NEJM199507063330104. [DOI] [PubMed] [Google Scholar]
- 13.Wilson P. D., Sherwood A. C., Palla K., Du J., Watson R., Norman J. T. Reversed polarity of Na+-K+-ATPase: mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am. J. Physiol. 1991;260:420–430. doi: 10.1152/ajprenal.1991.260.3.F420. [DOI] [PubMed] [Google Scholar]
- 14.Nadasdy T., Laszik Z., Lajoie G., Blick K. E., Wheeler D. E., Silva F. G. Proliferative activity of cyst epithelium in human renal cystic diseases. J. Am. Soc. Nephrol. 1995;5:1462–1468. doi: 10.1681/ASN.V571462. [DOI] [PubMed] [Google Scholar]
- 15.Wilson P. D., Geng L., Li X., Burrow C. R. The PKD1 gene product, “polycystin-1”, is a tyrosine-phosphorylated protein that colocalizes with α2β1-integrin in focal clusters in adherent renal epithelia. Lab. Invest. 1999;79:1311–1323. [PubMed] [Google Scholar]
- 16.Reeders S. T., Breuning M. H., Davies K. E., Nicholls R. D., Jarman A. P., Higgs D. R., Pearson P. L., Weatherall D. J. A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature (London) 1985;317:542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- 17.Mochizuki T., Wu G., Hayashi T., Xenophontos S. L., Veldhuisen B., Saris J. J., Reynolds D. M., Cai Y., Gabow P. A., Pierides A., et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 18.Clapham D. E. TRP channels as cellular sensors. Nature (London) 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 19.Wilson P. D. Polycystic kidney disease. N. Engl. J. Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 20.Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 21.Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cserzo M., Wallin E., Simon I., von Heijne G., Elofsson A. Prediction of transmembrane α-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 1997;10:673–676. doi: 10.1093/protein/10.6.673. [DOI] [PubMed] [Google Scholar]
- 23.Craven R. A., Griffiths D. J., Sheldrick K. S., Randall R. E., Hagan I. M., Carr A. M. Vectors for the expression of tagged proteins in Schizosaccharomyces pombe. Gene. 1998;221:59–68. doi: 10.1016/s0378-1119(98)00434-x. [DOI] [PubMed] [Google Scholar]
- 24.Forsburg S. L. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiely J., Haase S. B., Russell P., Leatherwood J. Functions of fission yeast orp2 in DNA replication and checkpoint control. Genetics. 2000;154:599–607. doi: 10.1093/genetics/154.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano K., Arai R., Mabuchi I. The small GTP-binding protein Rho 1 is a multifunctional protein that regulates actin localization, cell polarity, and septum formation in the fission yeast Schizosaccharomyces pombe. Genes Cells. 1997;2:679–694. doi: 10.1046/j.1365-2443.1997.1540352.x. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka N., Takegawa K. Functional characterization of Gms1p/UDP-galactose transporter in Schizosaccharomyces pombe. Yeast. 2001;18:745–757. doi: 10.1002/yea.725. [DOI] [PubMed] [Google Scholar]
- 28.Ohi R., Feoktistova A., Gould K. L. Construction of vectors and a genomic library for use with his3-deficient strains of Schizosaccharomyces pombe. Gene. 1996;174:315–318. doi: 10.1016/0378-1119(96)00085-6. [DOI] [PubMed] [Google Scholar]
- 29.Alloush H. M., Lopez-Ribot J. L., Masten B. J., Chaffin W. L. 3-Phosphoglycerate kinase: a glycolytic enzyme protein present in the cell wall of Candida albicans. Microbiology. 1997;143:321–330. doi: 10.1099/00221287-143-2-321. [DOI] [PubMed] [Google Scholar]
- 30.Sternberg P. W., Barr M. M. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature (London) 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 31.Watnick T. J., Jin Y., Matunis E., Kernan M. J., Montell C. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr. Biol. 2003;13:2179–2184. doi: 10.1016/j.cub.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Sun Z., Amsterdam A., Pazour G. J., Cole D. J., Miller M. S., Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X. L., Batiza A. F., Loukin S. H., Palmer C. P., Kung C., Saimi Y. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7105–7110. doi: 10.1073/pnas.1230540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer C. P., Zhou X. L., Lin J., Loukin S. H., Kung C., Saimi Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7801–7805. doi: 10.1073/pnas.141036198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ketchum K. A., Joiner W. J., Sellers A. J., Kaczmarek L. K., Goldstein S. A. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature (London) 1995;376:690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- 36.Bok J. W., Sone T., Silverman-Gavrila L. B., Bowring F. J., Catcheside D. E., Griffiths A. J. Structure and function analysis of the calcium-related gene spray in Neurospora crassa. Fungal Genet. Biol. 2001;32:145–158. doi: 10.1006/fgbi.2000.1259. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S. S., Ton V. K., Beaudry V., Rulli S., Cunningham K., Rao R. Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. J. Biol. Chem. 2003;278:28831–28839. doi: 10.1074/jbc.M303300200. [DOI] [PubMed] [Google Scholar]
- 38.Scheffers M. S., Le H., van der Bent P., Leonhard L., Prins F., Spruit L., Breuning M. H., de Heer E., Peters D. J. Distinct subcellular expression of endogenous polycystin-2 in the plasma membrane and Golgi apparatus of MDCK cells. Hum. Mol. Genet. 2002;11:59–67. doi: 10.1093/hmg/11.1.59. [DOI] [PubMed] [Google Scholar]
- 39.Walker S. A., Lockyer P. J., Cullen P. J. The Ras binary switch: an ideal processor for decoding complex Ca2+ signals? Biochem. Soc. Trans. 2003;31:966–969. doi: 10.1042/bst0310966. [DOI] [PubMed] [Google Scholar]
- 40.Walker S. A., Cullen P. J., Taylor J. A., Lockyer P. J. Control of Ras cycling by Ca2+ FEBS Lett. 2003;546:6–10. doi: 10.1016/s0014-5793(03)00412-5. [DOI] [PubMed] [Google Scholar]
- 41.Arellano M., Coll P. M., Yang W., Duran A., Tamanoi F., Perez P. Characterization of the geranylgeranyl transferase type I from Schizosaccharomyces pombe. Mol. Microbiol. 1998;29:1357–1367. doi: 10.1046/j.1365-2958.1998.01009.x. [DOI] [PubMed] [Google Scholar]
- 42.Inoue S. B., Qadota H., Arisawa M., Watanabe T., Ohya Y. Prenylation of Rho1p is required for activation of yeast 1,3-β-glucan synthase. J. Biol. Chem. 1999;274:38119–38124. doi: 10.1074/jbc.274.53.38119. [DOI] [PubMed] [Google Scholar]
- 43.Zhou X. L., Kung C. A mechanosensitive ion channel in Schizosaccharomyces pombe. EMBO J. 1992;11:2869–2875. doi: 10.1002/j.1460-2075.1992.tb05355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J., et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 45.Scheffers M. S., van der Bent P., van de Wal A., van Eendenburg J., Breuning M. H., de Heer E., Peters D. J. Altered distribution and co-localization of polycystin-2 with polycystin-1 in MDCK cells after wounding stress. Exp. Cell. Res. 2004;292:219–230. doi: 10.1016/j.yexcr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 46.Stokes N. R., Murray H. D., Subramaniam C., Gourse R. L., Louis P., Bartlett W., Miller S., Booth I. A role for mechanosensitive channels in survival of stationary phase: regulation of channel expression by RpoS. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15959–15964. doi: 10.1073/pnas.2536607100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hariharan I. K., Haber D. A. Yeast, flies, worms, and fish in the study of human disease. N. Engl. J. Med. 2003;348:2457–2463. doi: 10.1056/NEJMon023158. [DOI] [PubMed] [Google Scholar]
- 48.Barr M. M. Super models. Physiol. Genomics. 2003;13:15–24. doi: 10.1152/physiolgenomics.00075.2002. [DOI] [PubMed] [Google Scholar]
- 49.Miller C. Ion channel surprises: prokaryotes do it again! Neuron. 2000;25:7–9. doi: 10.1016/s0896-6273(00)80865-x. [DOI] [PubMed] [Google Scholar]