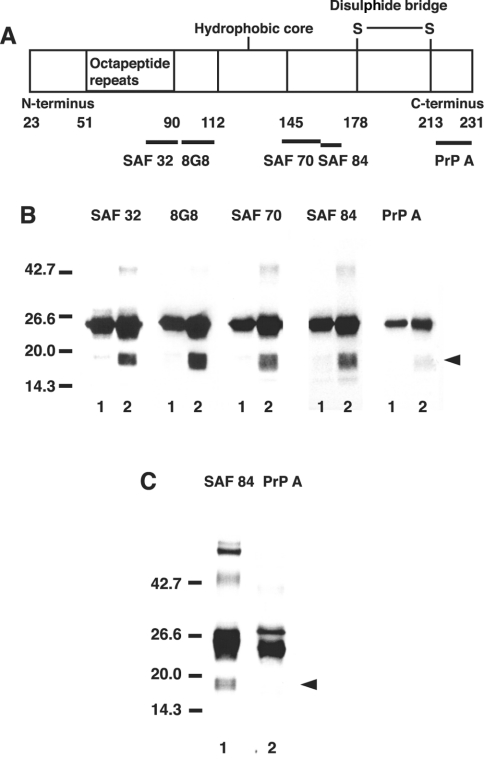
Figure 6. Production of C-terminally truncated species from copper-loaded PrP23–231 in the presence of dopamine.
(A) Diagrammatic presentation of the PrP regions recognized by the antibodies employed in the present study. The numbers represent the position of amino acid residue of the primary translation product. (B) Western blot immunoassay for copper-loaded PrP23–231. Mixtures containing 50 mM Mes (pH 7.5) and 1 μM copper-loaded PrP23–231 (containing 5.0 μM copper) were incubated in the absence (lanes 1) or presence of 10 μM dopamine (lanes 2) at 37 °C for 30 min. PrP was detected by SDS/PAGE, followed by immunoblotting with antibodies SAF 32, 8G8, SAF 70, SAF 84 and PrP A. Molecular masses in kDa are indicated on the left. (C) Immunoprecipitation of PrP. The complete reaction mixture was incubated as above, and anti-PrP monoclonal antibody (SAF 32) was added and allowed to stand for 1 h at room temperature. After incubation for 1 h with Protein G–Sepharose 4B, the beads were pelleted and washed extensively with 50 mM Mes buffer (pH 7.5) containing 0.15 M NaCl. The washed beads were mixed with 2× gel-loading buffer and heated at 95 °C for 5 min. PrP was detected by SDS/PAGE, followed by immunoblotting with antibodies SAF 84 (lane 1) and PrP A (lane 2). Molecular masses in kDa are indicated on the left side.