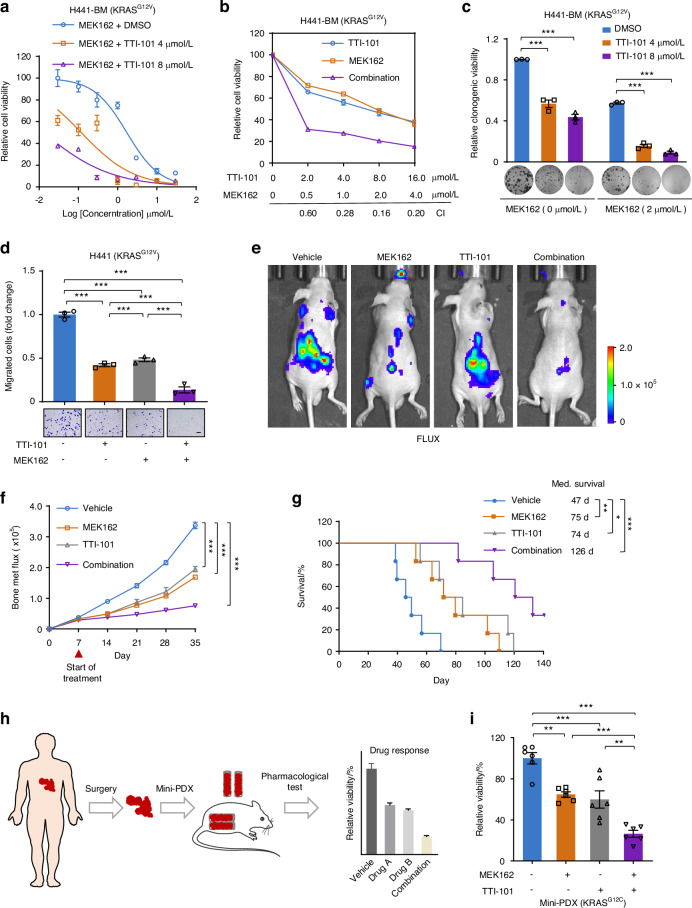
Fig. 8.
STAT3i sensitizes KRAS-mutant lung cancer bone metastasis to MEK inhibitor. a TTI-101 enhanced the inhibition efficacy of MEK162 in H441-BM cells. Combination refers to MEK162 (2 μmol/L) plus TTI-101 (4 μmol/L) cotreatment. Cells were treated with various concentrations of indicated inhibitors for 72 h. b Synergistic interaction between MEK162 (2 μmol/L) and TTI-101 (4 μmol/L) in H441-BM cells. Cells were treated with various concentrations of indicated inhibitors for 72 h. c, d TTI-101 (4 μmol/L) treatment increased MEK162 (2 μmol/L) mediated cytotoxicity. Cells treated with indicated inhibitors. The relative clonogenic viability (c) and migration ability (d) was normalized to vehicle-treated control. Scale bars, 50 μm. e Representative IVIS bioluminescence imaging of H441 cells injected into BALB/c nude mice (6 weeks) via intracardiac injection at day 35. After 7 days intracardiac injection, mice were treated with vehicle, TTI-101 (25 mg/kg), MEK162 (25 mg/kg) or combination for an additional 28 days (n = 6 per group). f The growth of bone metastasis in (e) was quantified. g Survival curves for indicated mice (n = 6 per group). h Scheme of the mini-PDX models. i The relative cell viability of mini-PDX treated with TTI-101 (25 mg/kg), MEK162 (25 mg/kg) or combination treatment for 7 days, and normalized to vehicle treatment (n = 6 per group). Data in (c, d) represent three technical replicates, representative of three independent experiments with similar results. Data in (a, b, c, d, f) shown as mean ± s.e.m. Panels (c, d, i) were performed one-way ANOVA with Tukey’s multiple comparison test, (f) performed two-way ANOVA with Sidak’s multiple comparisons test, and (g) using log-rank test, *P < 0.05, **P < 0.01, ***P < 0.001