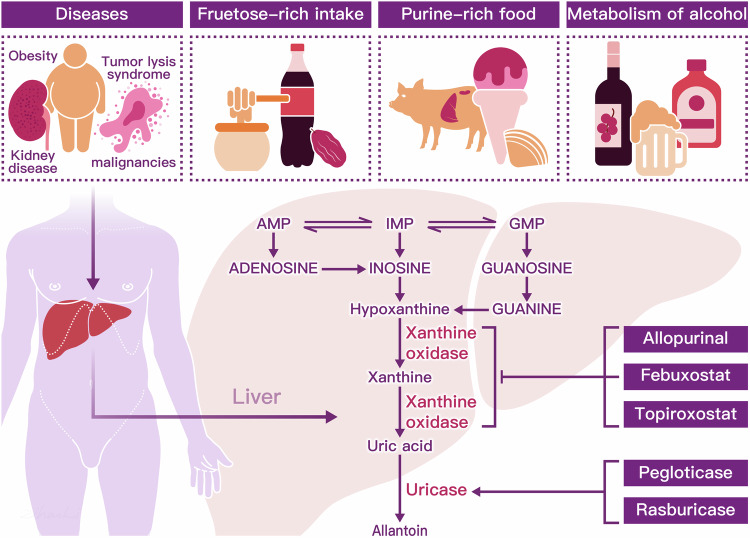
Fig. 2.
Consumption of purine-rich meats such as beef, pork, lamb, and seafood like oysters, shrimp, and tuna, as well as dietary fructose, are known to elevate uric acid (UA) production. Additionally, alcohol metabolism from beer and distilled spirits, along with certain medical conditions such as tumor lysis syndrome and obesity, pose increased risks for hyperuricemia. Hepatic metabolism of uric acid involves the sequential processing of purine nucleotides, including adenosine monophosphate (AMP), guanosine monophosphate (GMP), and inosine monophosphate (IMP).66 IMP plays a pivotal role as a key intermediate in purine nucleotide biosynthesis, serving as a precursor for the synthesis of both adenosine monophosphate (AMP) and guanosine monophosphate (GMP). Moreover, IMP can be enzymatically deaminated by IMP dehydrogenase, leading to the formation of inosine. Inosine, in turn, can undergo phosphorylation to become hypoxanthine. Hypoxanthine undergoes oxidative reactions catalyzed by xanthine oxidase (XOD), resulting in the production of xanthine. Xanthine can further undergo oxidation reactions, also catalyzed by XOD, ultimately leading to the formation of uric acid from xanthine.118 However, xanthine oxidase inhibitors, such as allopurinol, febuxostat, and topiroxostat, serve as first-line therapies by effectively reducing the production of uric acid from both endogenous and dietary purine sources. In the final step of purine metabolism, the enzyme uricase converts uric acid into allantoin, a highly soluble compound. While humans lack the uricase enzyme, animals naturally possess it. The therapeutic agents pegloticase and rasburicase are recombinant forms of uricase, designed to facilitate the breakdown of uric acid in humans