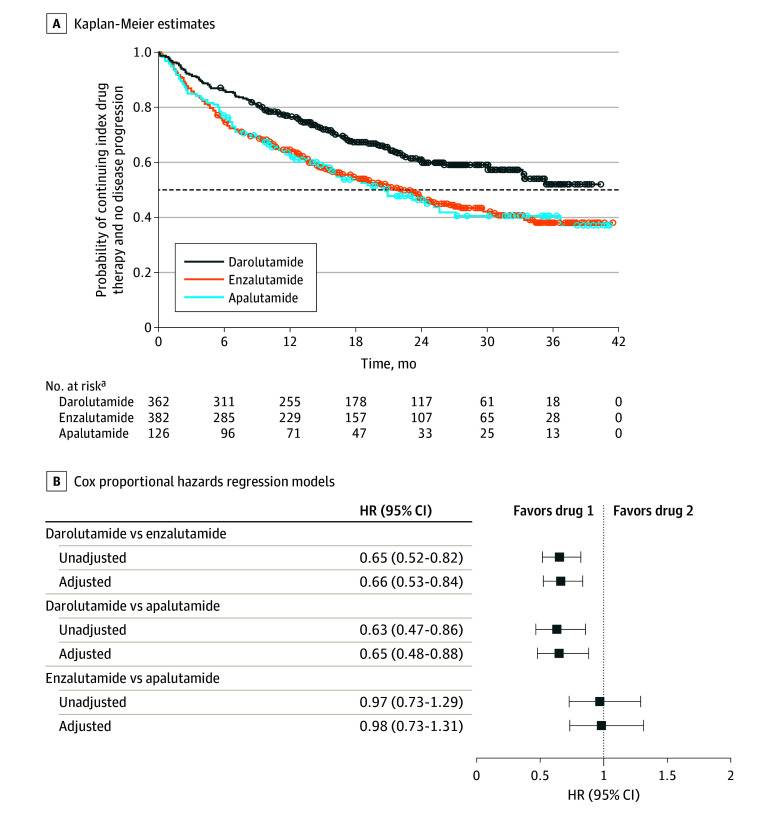
Figure 1. Composite End Point of Time to Initial Androgen Receptor Inhibitor (ARI) Therapy Discontinuation or Progression to Metastatic Castration-Resistant Prostate Cancer (mCRPC).
Composite end point was defined as the earliest occurrence of evidence of stopping initial ARI treatment, switch to another ARI, evidence of metastatic lesion(s), diagnosis of mCRPC, drug treatment initiated specifically for mCRPC (ie, abiraterone acetate, docetaxel, cabazitaxel, sipuleucel-T, mitoxantrone hydrochloride, or radium RA 223 dichloride), or death. The dashed line indicates posterior probability of 0.5. A total of 791 patients (328 receiving darolutamide, 341 receiving enzalutamide, and 122 receiving apalutamide) were included in the Cox proportional hazards regression model after excluding 79 patients with unknown insurance coverage and/or missing baseline prostate-specific antigen (PSA) values. The following observed baseline characteristics were included in the adjusted Cox proportional hazards regression models: age group at index (≤74, 75-84, or ≥85 years), race (Black or African American, White, or other or unknown), insurance coverage (commercial or public), index period (2019-2020 or 2021-2022), baseline PSA group (<2, 2 to <10, or ≥10 ng/mL), baseline PSA doubling time group (≤6, >6 to ≤10, or >10 months or missing), time from non-mCRPC diagnosis to index date (months), and Gleason score at initial prostate cancer diagnosis (4-7, 8-10, or missing). HR indicates hazard ratio.
aNumbers of patients at risk were calculated at the start of each time point.