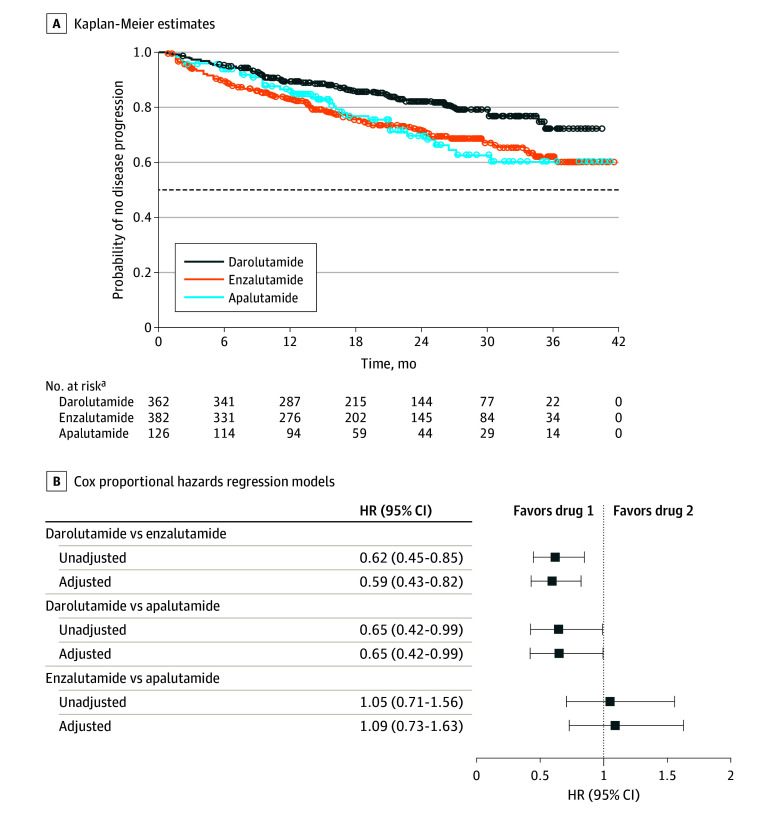
Figure 4. Time to Progression to Metastatic Castration-Resistant Prostate Cancer (mCRPC).
Progression to mCRPC was defined as the earliest occurrence of a clear diagnosis of mCRPC, evidence of metastasis in patient medical records or radiology reports, or drug treatment initiated specifically for mCRPC (ie, abiraterone acetate, docetaxel, cabazitaxel, sipuleucel-T, mitoxantrone hydrochloride, or radium RA 223 dichloride). The dashed line indicates posterior probability of 0.5. A total of 791 patients (328 receiving darolutamide, 341 receiving enzalutamide, and 122 receiving apalutamide) were included in the Cox proportional hazards regression model after excluding 79 patients with unknown insurance coverage and/or missing baseline prostate-specific antigen (PSA) values. The following observed baseline characteristics were included in the adjusted Cox proportional hazards regression models: age group at index (≤74, 75-84, or ≥85 years), race (Black or African American, White, or other or unknown), insurance coverage (commercial or public), index period (2019-2020 or 2021-2022), baseline PSA group (<2, 2 to <10, or ≥10 ng/mL), baseline PSA doubling time group (≤6, >6 to ≤10, or >10 months or missing), time from non-mCRPC diagnosis to index date (months), and Gleason score at initial prostate cancer diagnosis (4-7, 8-10, or missing).
aNumbers of patients at risk were calculated at the start of each time point.