Abstract
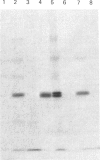
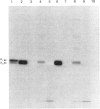
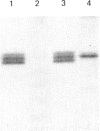
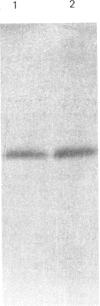
In muscle, it has been established that guanosine 5'-[gamma-thio]triphosphate (GTP[S]), a non-hydrolysable GTP analogue, elicits a rise in tension in chemically skinned fibres, and that pretreatment with Bordetella pertussis toxin (PTX) decreases GTP[S]-induced tension development [Di Virgilio, Salviati, Pozzan & Volpe (1986) EMBO J. 5, 259-262]. In the present study, G-proteins were analysed by PTX-catalysed ADP-ribosylation and by immunoblotting experiments at cellular and subcellular levels. First, the nature of the G-proteins present in neural and aneural zones of rat diaphragm muscle was investigated. PTX, known to catalyse the ADP-ribosylation of the alpha subunit of several G-proteins, was used to detect G-proteins. Three sequential extractions (low-salt-soluble, detergent-soluble and high-salt-soluble) were performed, and PTX was found to label two substrates of 41 and 40 kDa only in the detergent-soluble fraction. The addition of pure beta gamma subunits of G-proteins to the low-salt-soluble extract did not provide a way to detect PTX-catalysed ADP-ribosylation of G-protein alpha subunits in this hydrophilic fraction. In neural as well as in aneural zones, the 39 kDa PTX substrate, very abundant in the nervous system (Go alpha), was not observed. We then studied the nature of the G alpha subunits present in membranes from transverse tubules (T-tubules) purified from rabbit skeletal muscle. Only one 40 kDa PTX substrate was found in T-tubules, known to be the key element of excitation-contraction coupling. The presence of a G-protein in T-tubule membranes was further confirmed by the immunoreactivity detected with an anti-beta-subunit antiserum. A 40 kDa protein was also detected in T-tubule membranes with an antiserum raised against a purified bovine brain Go alpha. The presence of two PTX substrates (41 and 40 kDa) in equal amounts in total muscle extracts, compared with only one (40 kDa) found in purified T-tubule membranes, suggests that this 40 kDa PTX substrate might be involved in excitation-contraction coupling.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Bon S., Massoulié J. Collagen-tailed and hydrophobic components of acetylcholinesterase in Torpedo marmorata electric organ. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4464–4468. doi: 10.1073/pnas.77.8.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsotto M., Barhanin J., Norman R. I., Lazdunski M. Purification of the dihydropyridine receptor of the voltage-dependent Ca2+ channel from skeletal muscle transverse tubules using (+) [3H]PN 200-110. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1357–1366. doi: 10.1016/0006-291x(84)91241-5. [DOI] [PubMed] [Google Scholar]
- Brabet P., Dumuis A., Sebben M., Pantaloni C., Bockaert J., Homburger V. Immunocytochemical localization of the guanine nucleotide-binding protein Go in primary cultures of neuronal and glial cells. J Neurosci. 1988 Feb;8(2):701–708. doi: 10.1523/JNEUROSCI.08-02-00701.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Salviati G., Pozzan T., Volpe P. Is a guanine nucleotide-binding protein involved in excitation-contraction coupling in skeletal muscle? EMBO J. 1986 Feb;5(2):259–262. doi: 10.1002/j.1460-2075.1986.tb04207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey B. F., Pyun H. Y., Williamson K. C., Navarro J. Identification and purification of a novel G protein from neutrophils. FEBS Lett. 1987 Jul 27;219(2):289–292. doi: 10.1016/0014-5793(87)80237-5. [DOI] [PubMed] [Google Scholar]
- Evans T., Martin M. W., Hughes A. R., Harden T. K. Guanine nucleotide-sensitive, high affinity binding of carbachol to muscarinic cholinergic receptors of 1321N1 astrocytoma cells is insensitive to pertussis toxin. Mol Pharmacol. 1985 Jan;27(1):32–37. [PubMed] [Google Scholar]
- Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983 May 25;258(10):6086–6092. [PubMed] [Google Scholar]
- Gierschik P., Falloon J., Milligan G., Pines M., Gallin J. I., Spiegel A. Immunochemical evidence for a novel pertussis toxin substrate in human neutrophils. J Biol Chem. 1986 Jun 15;261(17):8058–8062. [PubMed] [Google Scholar]
- Gierschik P., Sidiropoulos D., Spiegel A., Jakobs K. H. Purification and immunochemical characterization of the major pertussis-toxin-sensitive guanine-nucleotide-binding protein of bovine-neutrophil membranes. Eur J Biochem. 1987 May 15;165(1):185–194. doi: 10.1111/j.1432-1033.1987.tb11210.x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homburger V., Brabet P., Audigier Y., Pantaloni C., Bockaert J., Rouot B. Immunological localization of the GTP-binding protein Go in different tissues of vertebrates and invertebrates. Mol Pharmacol. 1987 Apr;31(4):313–319. [PubMed] [Google Scholar]
- Huff R. M., Neer E. J. Subunit interactions of native and ADP-ribosylated alpha 39 and alpha 41, two guanine nucleotide-binding proteins from bovine cerebral cortex. J Biol Chem. 1986 Jan 25;261(3):1105–1110. [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Katada T., Oinuma M., Kusakabe K., Ui M. A new GTP-binding protein in brain tissues serving as the specific substrate of islet-activating protein, pertussis toxin. FEBS Lett. 1987 Mar 23;213(2):353–358. doi: 10.1016/0014-5793(87)81521-1. [DOI] [PubMed] [Google Scholar]
- Katada T., Oinuma M., Ui M. Two guanine nucleotide-binding proteins in rat brain serving as the specific substrate of islet-activating protein, pertussis toxin. Interaction of the alpha-subunits with beta gamma-subunits in development of their biological activities. J Biol Chem. 1986 Jun 25;261(18):8182–8191. [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Malbon C. C., Mangano T. J., Watkins D. C. Heart contains two substrates (Mr = 40,000 and 41,000) for pertussis toxin-catalyzed ADP-ribosylation that co-purify with Ns. Biochem Biophys Res Commun. 1985 Apr 30;128(2):809–815. doi: 10.1016/0006-291x(85)90119-6. [DOI] [PubMed] [Google Scholar]
- Milligan G., Gierschik P., Spiegel A. M., Klee W. A. The GTP-binding regulatory proteins of neuroblastoma x glioma, NG108-15, and glioma, C6, cells. Immunochemical evidence of a pertussis toxin substrate that is neither Ni nor No. FEBS Lett. 1986 Jan 20;195(1-2):225–230. doi: 10.1016/0014-5793(86)80165-x. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Rapiejko P. J., Northup J. K., Evans T., Brown J. E., Malbon C. C. G-proteins of fat-cells. Role in hormonal regulation of intracellular inositol 1,4,5-trisphosphate. Biochem J. 1986 Nov 15;240(1):35–40. doi: 10.1042/bj2400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemblatt M., Hidalgo C., Vergara C., Ikemoto N. Immunological and biochemical properties of transverse tubule membranes isolated from rabbit skeletal muscle. J Biol Chem. 1981 Aug 10;256(15):8140–8148. [PubMed] [Google Scholar]
- Sternweis P. C. The purified alpha subunits of Go and Gi from bovine brain require beta gamma for association with phospholipid vesicles. J Biol Chem. 1986 Jan 15;261(2):631–637. [PubMed] [Google Scholar]
- Suki W. N., Abramowitz J., Mattera R., Codina J., Birnbaumer L. The human genome encodes at least three non-allellic G proteins with alpha i-type subunits. FEBS Lett. 1987 Aug 10;220(1):187–192. doi: 10.1016/0014-5793(87)80900-6. [DOI] [PubMed] [Google Scholar]
- Toutant M., Aunis D., Bockaert J., Homburger V., Rouot B. Presence of three pertussis toxin substrates and Go alpha immunoreactivity in both plasma and granule membranes of chromaffin cells. FEBS Lett. 1987 May 11;215(2):339–344. doi: 10.1016/0014-5793(87)80174-6. [DOI] [PubMed] [Google Scholar]
- Toutant M., Bockaert J., Homburger V., Rouot B. G-proteins in Torpedo marmorata electric organ. Differential distribution in pre- and post-synaptic membranes and synaptic vesicles. FEBS Lett. 1987 Sep 28;222(1):51–55. doi: 10.1016/0014-5793(87)80190-4. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]