Abstract
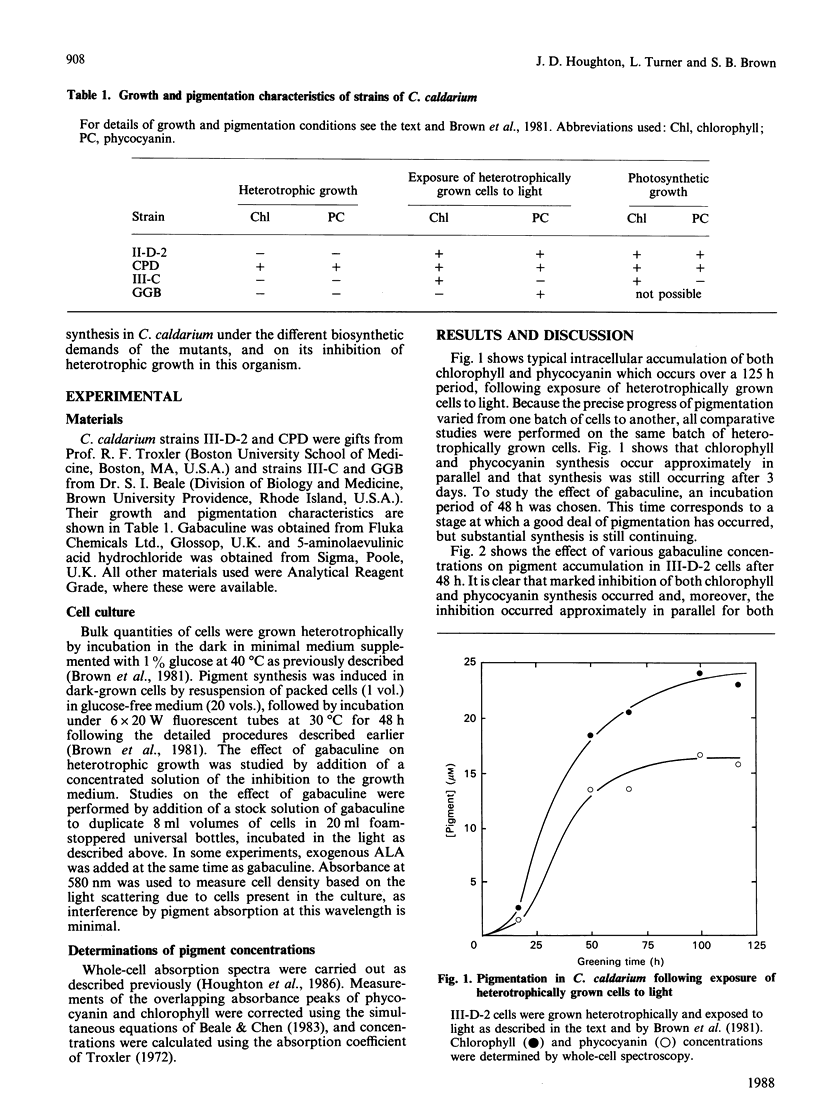
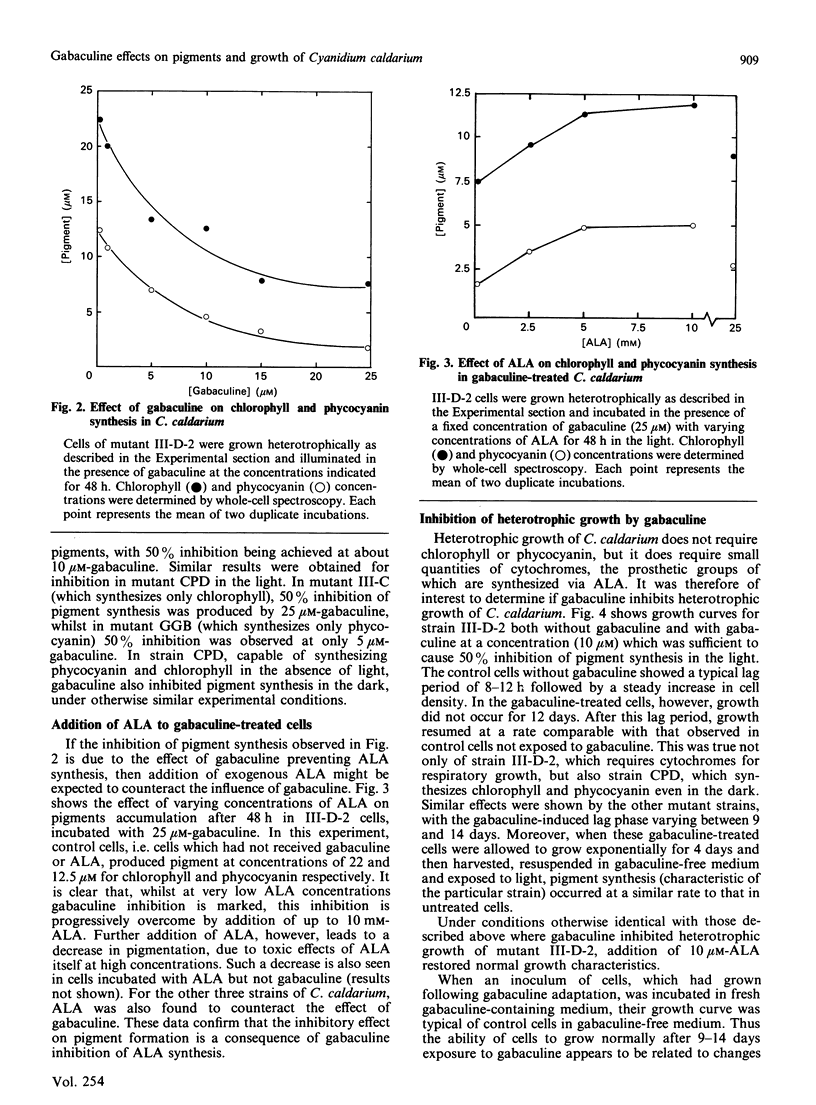
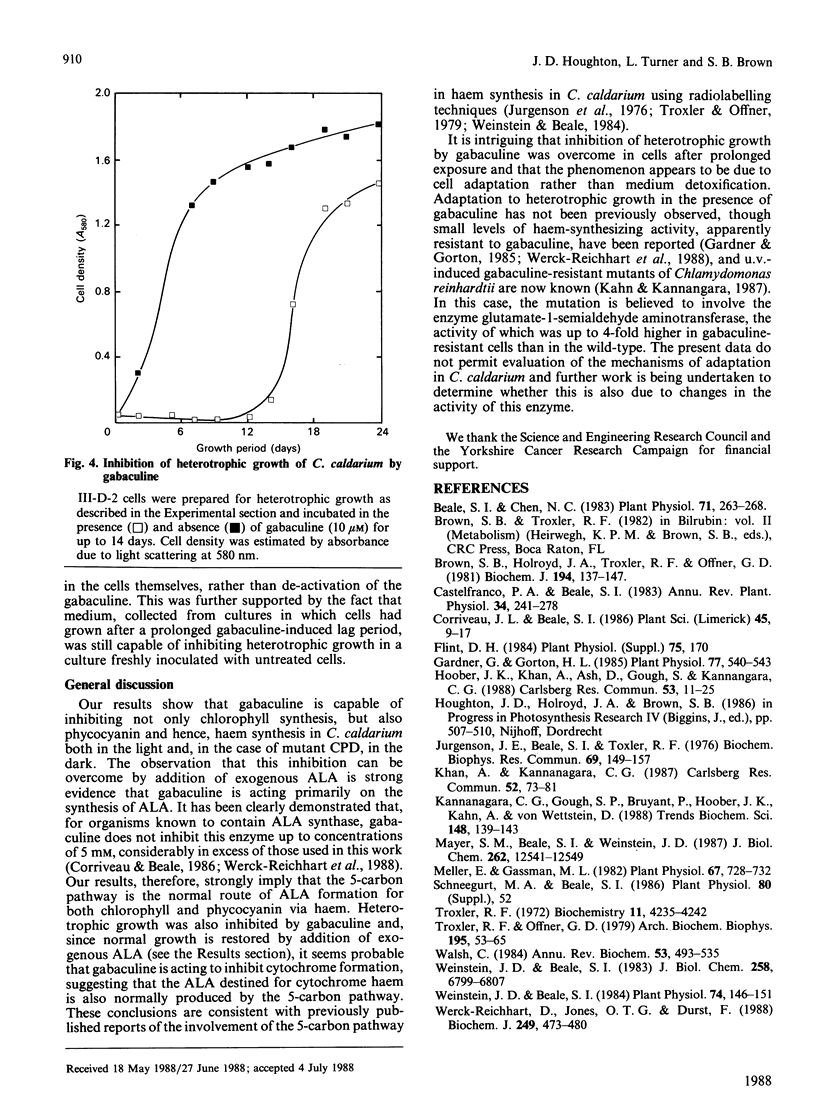
Pigment synthesis in four strains of the unicellular red alga Cyanidium caldarium with different pigment-synthesizing patterns was inhibited in the presence of gabaculine (3-amino-2,3-dihydrobenzoic acid). Parallel inhibition of light-induced chlorophyll and phycocyanin synthesis was observed in strain III-D-2, which only synthesizes pigments in the light. Similar parallel inhibition was observed in the dark in mutant CPD, which is able to synthesize chlorophyll and phycocyanin in the absence of light. Inhibition of pigment synthesis in all strains was overcome by addition of 5-aminolaevulinic acid. Inhibition of phycocyanin synthesis in mutant GGB (unable to synthesize chlorophyll) and inhibition of chlorophyll synthesis in mutant III-C (unable to synthesize phycocyanin) were also observed. Gabaculine also inhibited the heterotrophic growth of C. caldarium in the dark. However, inhibition was overcome after an extended lag period, following which cell growth proceeded at a similar rate to that of control cells not exposed to gabaculine. Heterotrophic growth in cells pre-exposed to gabaculine was not inhibited by subsequent exposure. Possible mechanisms for this adaptation are discussed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Chen N. C. N-Methyl Mesoporphyrin IX Inhibits Phycocyanin, but Not Chlorophyll Synthesis in Cyanidium caldarium. Plant Physiol. 1983 Feb;71(2):263–268. doi: 10.1104/pp.71.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Holroyd J. A., Troxler R. F., Offner G. D. Bile pigment synthesis in plants. Incorporation of haem into phycocyanobilin and phycobiliproteins in Cyanidium caldarium. Biochem J. 1981 Jan 15;194(1):137–147. doi: 10.1042/bj1940137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner G., Gorton H. L. Inhibition of phytochrome synthesis by gabaculine. Plant Physiol. 1985 Mar;77(3):540–543. doi: 10.1104/pp.77.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Kahn A., Ash D. E., Gough S., Kannangara C. G. Biosynthesis of delta-aminolevulinate in greening barley leaves. IX. Structure of the substrate, mode of gabaculine inhibition, and the catalytic mechanism of glutamate 1-semialdehyde aminotransferase. Carlsberg Res Commun. 1988;53(1):11–25. doi: 10.1007/BF02908411. [DOI] [PubMed] [Google Scholar]
- Jurgenson J. E., Beale S. I., Troxler R. F. Biosynthesis of delta-aminolevulinic acid in the unicellular rhodophyte, cyanidium caldarium. Biochem Biophys Res Commun. 1976 Mar 8;69(1):149–157. doi: 10.1016/s0006-291x(76)80285-9. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Gough S. P., Bruyant P., Hoober J. K., Kahn A., von Wettstein D. tRNA(Glu) as a cofactor in delta-aminolevulinate biosynthesis: steps that regulate chlorophyll synthesis. Trends Biochem Sci. 1988 Apr;13(4):139–143. doi: 10.1016/0968-0004(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Mayer S. M., Beale S. I., Weinstein J. D. Enzymatic conversion of glutamate to delta-aminolevulinic acid in soluble extracts of Euglena gracilis. J Biol Chem. 1987 Sep 15;262(26):12541–12549. [PubMed] [Google Scholar]
- Meller E., Gassman M. L. The effects of levulinic Acid and 4,6-dioxoheptanoic Acid on the metabolism of etiolated and greening barley leaves. Plant Physiol. 1981 Apr;67(4):728–732. doi: 10.1104/pp.67.4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. A. The Effects of N Nutrition on the Water Relations and Gas Exchange Characteristics of Wheat (Triticum aestivum L.). Plant Physiol. 1986 Jan;80(1):52–58. doi: 10.1104/pp.80.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F., Offner G. D. delta-Aminolevulinic acid synthesis in a Cyanidium caldarium mutant unable to make chlorophyll a and phycobiliproteins. Arch Biochem Biophys. 1979 Jun;195(1):53–65. doi: 10.1016/0003-9861(79)90326-6. [DOI] [PubMed] [Google Scholar]
- Troxler R. F. Synthesis of bile pigments in plants. Formation of carbon monoxide and phycocyanobilin in wild-type and mutant strains of the alga, Cyanidium caldarium. Biochemistry. 1972 Nov 7;11(23):4235–4242. doi: 10.1021/bi00773a007. [DOI] [PubMed] [Google Scholar]
- Walsh C. T. Suicide substrates, mechanism-based enzyme inactivators: recent developments. Annu Rev Biochem. 1984;53:493–535. doi: 10.1146/annurev.bi.53.070184.002425. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Biosynthesis of Protoheme and Heme a Precursors Solely from Glutamate in the Unicellular Red Alga Cyanidium caldarium. Plant Physiol. 1984 Jan;74(1):146–151. doi: 10.1104/pp.74.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis. J Biol Chem. 1983 Jun 10;258(11):6799–6807. [PubMed] [Google Scholar]
- Werck-Reichhart D., Jones O. T., Durst F. Haem synthesis during cytochrome P-450 induction in higher plants. 5-Aminolaevulinic acid synthesis through a five-carbon pathway in Helianthus tuberosus tuber tissues aged in the dark. Biochem J. 1988 Jan 15;249(2):473–480. doi: 10.1042/bj2490473. [DOI] [PMC free article] [PubMed] [Google Scholar]


