Abstract
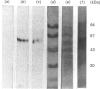
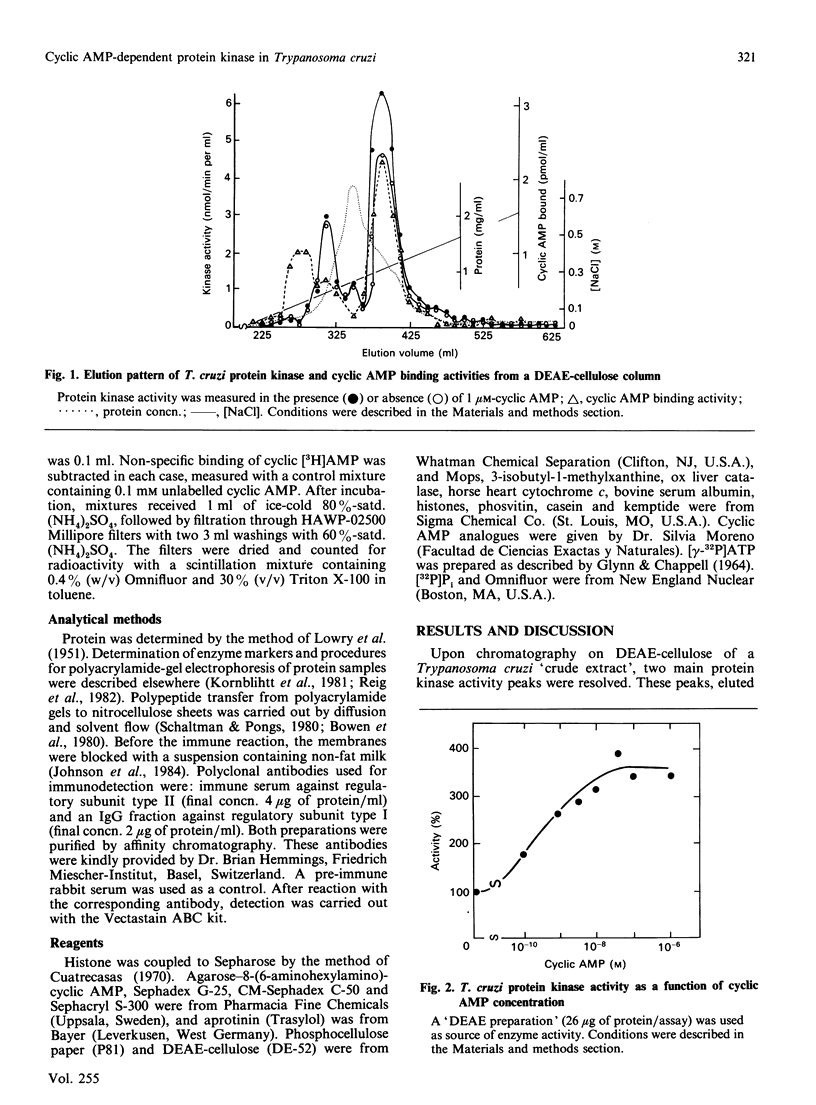
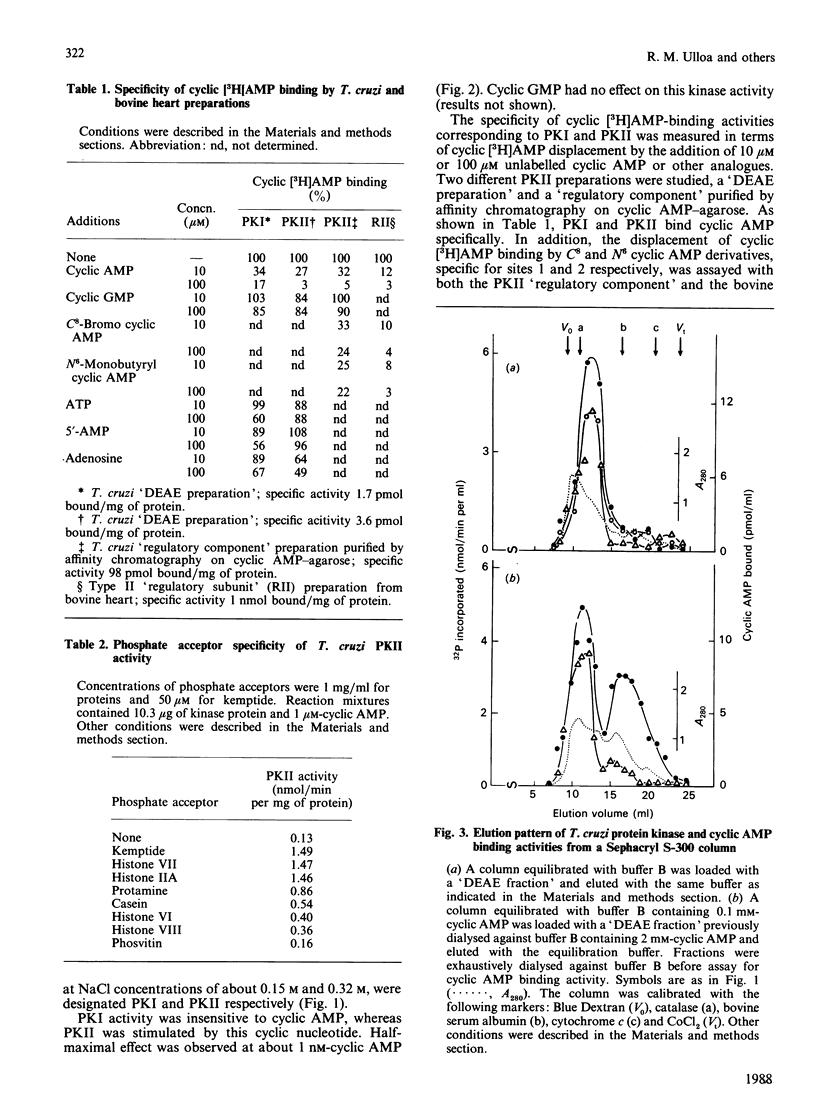
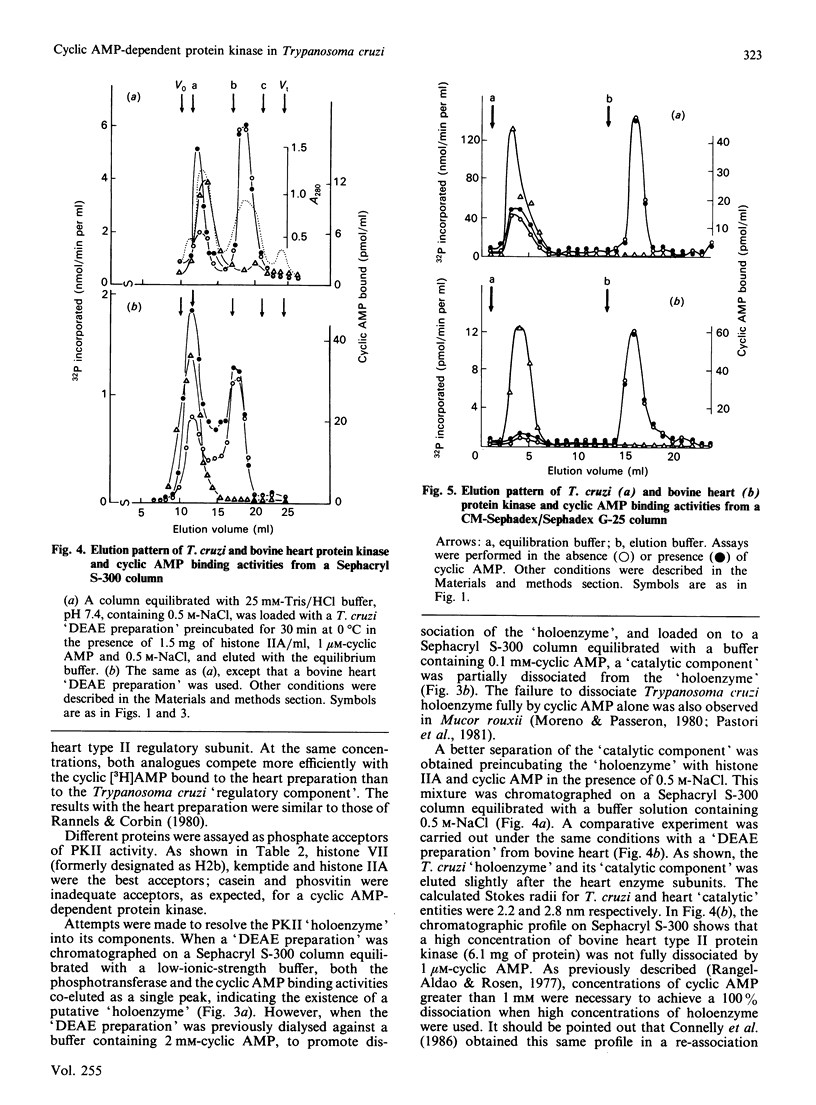
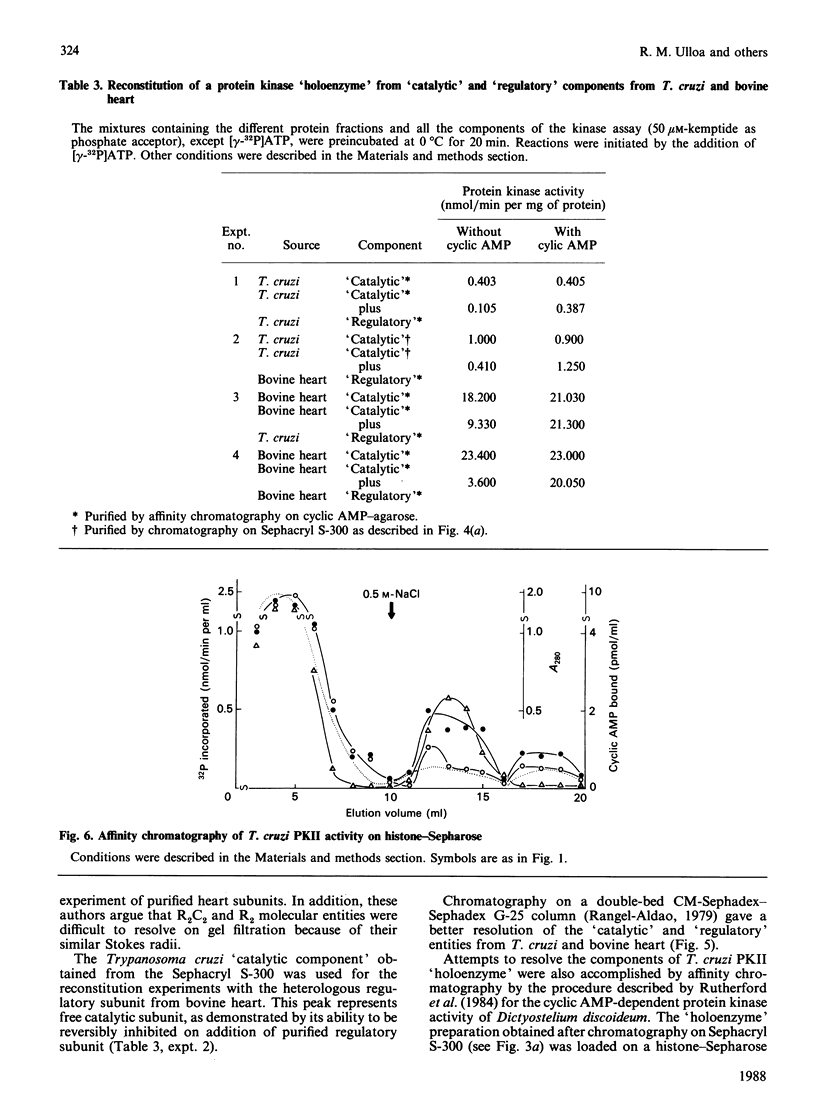
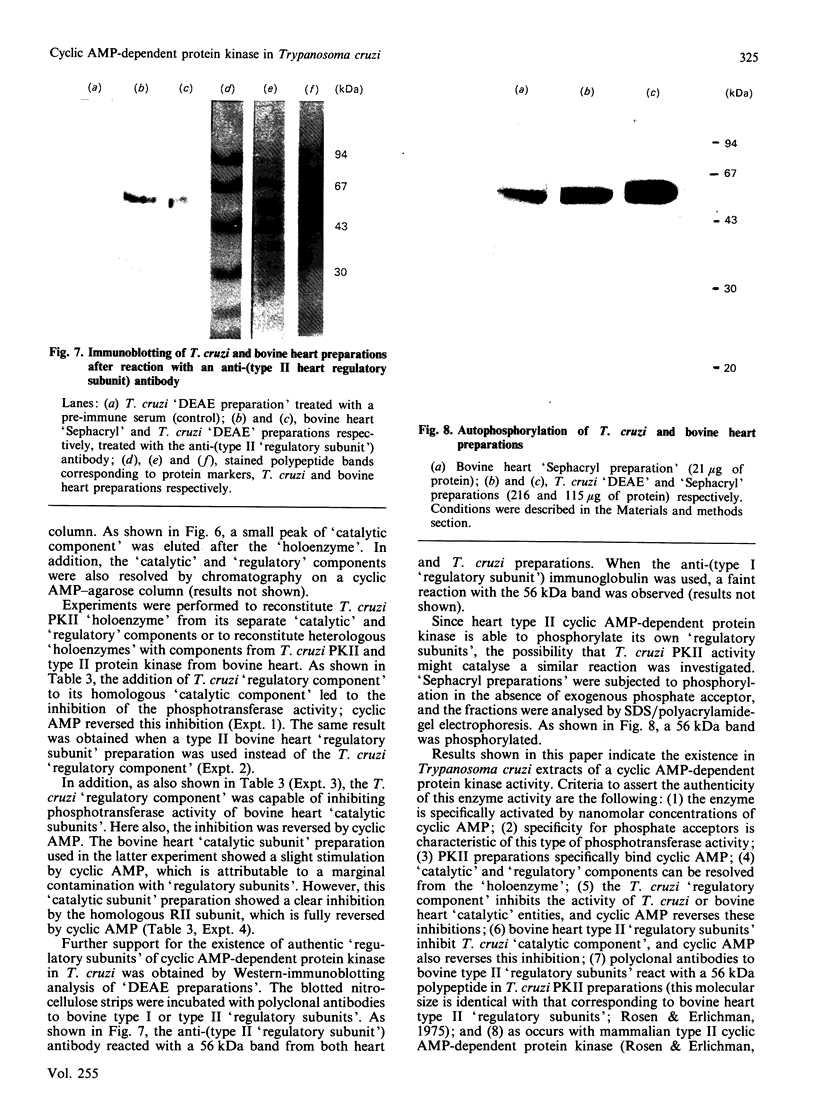
A cyclic AMP-dependent protein kinase activity from epimastigote forms of Trypanosoma cruzi was characterized. Cytosolic extracts were chromatographed on DEAE-cellulose columns, giving two peaks of kinase activity, which were eluted at 0.15 M- and 0.32 M-NaCl respectively. The second activity peak was stimulated by nanomolar concentrations of cyclic AMP. In addition, a cyclic AMP-binding protein co-eluted with the second kinase activity peak. Cyclic AMP-dependent protein kinase activity was further purified by gel filtration, affinity chromatography on histone-agarose and cyclic AMP-agarose, as well as by chromatography on CM-Sephadex. The enzyme ('holoenzyme') could be partially dissociated into two different components: 'catalytic' and 'regulatory'. The 'regulatory' component had specific binding for cyclic AMP, and it inhibited phosphotransferase activity of the homologous 'catalytic component' or of the 'catalytic subunit' from bovine heart. Cyclic AMP reversed these inhibitions. A 'holoenzyme preparation' was phosphorylated in the absence of exogenous phosphate acceptor and analysed by polyacrylamide-gel electrophoresis. A 56 kDa band was phosphorylated. The same preparation was analysed by Western blotting, by using polyclonal antibodies to the regulatory subunits of protein kinases type I or II. Both antibodies reacted with the 56 kDa band.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Bechtel P. J., Krebs E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly P. A., Hastings T. G., Reimann E. M. Identification of a ternary complex between cAMP and a trimeric form of cAMP-dependent protein kinase. J Biol Chem. 1986 Feb 15;261(5):2325–2330. [PubMed] [Google Scholar]
- Contreras V. T., Salles J. M., Thomas N., Morel C. M., Goldenberg S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol Biochem Parasitol. 1985 Sep;16(3):315–327. doi: 10.1016/0166-6851(85)90073-8. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dills W. L., Goodwin C. D., Lincoln T. M., Beavo J. A., Bechtel P. J., Corbin J. D., Krebs E. G. Purification of cyclic nucleotide receptor proteins by cyclic nucleotide affinity chromatography. Adv Cyclic Nucleotide Res. 1979;10:199–217. [PubMed] [Google Scholar]
- Doskeland S. O., Ueland P. M. Binding proteins for adenosine 3':5'-cyclic monophosphate in bovine adrenal cortex. Biochem J. 1977 Sep 1;165(3):561–573. doi: 10.1042/bj1650561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak J. A. The natural heterogeneity of Trypanosoma cruzi: biological and medical implications. J Cell Biochem. 1984;24(4):357–371. doi: 10.1002/jcb.240240406. [DOI] [PubMed] [Google Scholar]
- Frasch A. C., Goijman S. G., Cazzulo J. J., Stoppani A. O. Constant and variable regions in DNA mini-circles from Trypanosoma cruzi and Trypanosoma rangeli: application to species and stock differentiation. Mol Biochem Parasitol. 1981 Dec;4(3-4):163–170. doi: 10.1016/0166-6851(81)90015-3. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Flawia M. M., Torres H. N. Manganese ion dependent adenylate cyclase activity in rat testes: purification and properties. Biochemistry. 1981 Mar 3;20(5):1262–1267. doi: 10.1021/bi00508a033. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mattei D. M., Goldenberg S., Morel C. Biochemical strain characterization of Trypanosoma cruzi by restriction endonuclease cleavage of kinetoplast-DNA. FEBS Lett. 1977 Mar 1;74(2):264–268. doi: 10.1016/0014-5793(77)80860-0. [DOI] [PubMed] [Google Scholar]
- Miles M. A., Cibulskis R. E. Zymodeme characterization of Trypanosoma cruzi. Parasitol Today. 1986 Apr;2(4):94–97. doi: 10.1016/0169-4758(86)90037-2. [DOI] [PubMed] [Google Scholar]
- Miles M. A., Toye P. J., Oswald S. C., Godfrey D. G. The identification by isoenzyme patterns of two distinct strain-groups of Trypanosoma cruzi, circulating independently in a rural area of Brazil. Trans R Soc Trop Med Hyg. 1977;71(3):217–225. doi: 10.1016/0035-9203(77)90012-8. [DOI] [PubMed] [Google Scholar]
- Morel C. M., Deane M. P., Gonçalves A. M. The complexity of Trypanosoma cruzi populations revealed by schizodeme analysis. Parasitol Today. 1986 Apr;2(4):97–101. doi: 10.1016/0169-4758(86)90038-4. [DOI] [PubMed] [Google Scholar]
- Moreno S., Passeron S. Further studies on cyclic adenosine 3':5'-monophosphate protein kinase from dimorphic fungus Mucor rouxii. Arch Biochem Biophys. 1980 Feb;199(2):321–330. doi: 10.1016/0003-9861(80)90287-8. [DOI] [PubMed] [Google Scholar]
- Pastori R. L., Kerner N., Moreno S., Passeron S. cAMP-dependent protein kinase from Mucor rouxii: physical evidence of a ternary complex holoenzyme-cAMP. Biochem Biophys Res Commun. 1981 Jul 30;101(2):663–671. doi: 10.1016/0006-291x(81)91310-3. [DOI] [PubMed] [Google Scholar]
- Rangel-Aldao R., Rosen O. M. Effect of cAMP and ATP on the reassociation of phosphorylated and nonphosphorylated subunits of the cAMP-dependent protein kinase from bovine cardiac muscle. J Biol Chem. 1977 Oct 25;252(20):7140–7145. [PubMed] [Google Scholar]
- Rangel-Aldao R., Tovar G., Ledezma de Ruiz M. The cAMP receptor protein of Trypanosoma cruzi. J Biol Chem. 1983 Jun 10;258(11):6979–6983. [PubMed] [Google Scholar]
- Rannels S. R., Beasley A., Corbin J. D. Regulatory subunits of bovine heart and rabbit skeletal muscle cAMP-dependent protein kinase isozymes. Methods Enzymol. 1983;99:55–62. doi: 10.1016/0076-6879(83)99040-7. [DOI] [PubMed] [Google Scholar]
- Rannels S. R., Corbin J. D. Two different intrachain cAMP binding sites of cAMP-dependent protein kinases. J Biol Chem. 1980 Aug 10;255(15):7085–7088. [PubMed] [Google Scholar]
- Reig J. A., Kornblihtt A. R., Flawiá M. M., Torres H. N. Soluble adenylate cyclase activity in Neurospora crassa. Biochem J. 1982 Oct 1;207(1):43–49. doi: 10.1042/bj2070043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Rangel-Aldao R., Sarkar D., Erlichman J., Fleischer N. Characterization and comparison of membrane-associated and cytosolic cAMP-dependent protein kinases. Physicochemical and immunological studies on bovine cerebral cortex protein kinases. J Biol Chem. 1979 May 25;254(10):3797–3805. [PubMed] [Google Scholar]
- Rutherford C. L., Vaughan R. L., Cloutier M. J., Ferris D. K., Brickey D. A. Chromatographic behavior of cyclic AMP dependent protein kinase and its subunits from Dictyostelium discoideum. Biochemistry. 1984 Sep 25;23(20):4611–4617. doi: 10.1021/bi00315a015. [DOI] [PubMed] [Google Scholar]
- Schaltmann K., Pongs O. A simple procedure for blotting of proteins to study antibody specificity and antigen structure. Hoppe Seylers Z Physiol Chem. 1980;361(2):207–210. [PubMed] [Google Scholar]
- Téllez-Iñn M. T., Ulloa R. M., Torruella M., Torres H. N. Calmodulin and Ca2+-dependent cyclic AMP phosphodiesterase activity in Trypanosoma cruzi. Mol Biochem Parasitol. 1985 Nov;17(2):143–153. doi: 10.1016/0166-6851(85)90013-1. [DOI] [PubMed] [Google Scholar]
- Walter R. D. Multiple protein kinases from Trypanosoma gambiense. Hoppe Seylers Z Physiol Chem. 1978 May;359(5):601–606. doi: 10.1515/bchm.1978.359.1.601. [DOI] [PubMed] [Google Scholar]
- Wynne de Martini G. J., Abramo Orrego L., de Rissio A. M., Alvarez M., Mujica L. P. Cultivo de Trypanosoma cruzi en un médio monofásico. Su aplicación en cultivos en gran escala en procesos de fermentación. Medicina (B Aires) 1980;40 (Suppl 1):109–114. [PubMed] [Google Scholar]