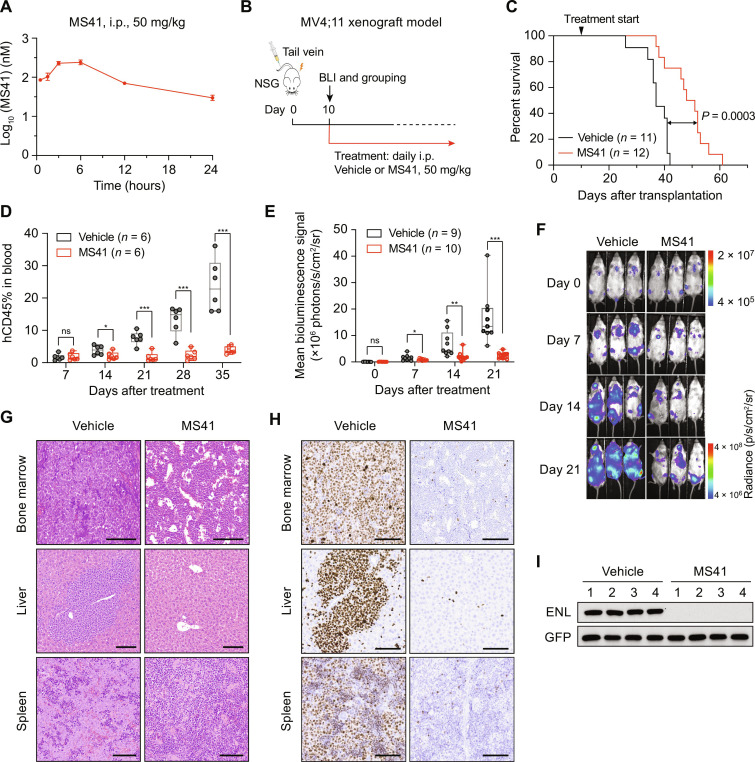
Fig. 6. MS41 suppresses leukemia progression in a xenograft model of MLL-r leukemia.
(A) Plasma concentrations of MS41 over a 24-hour period in mice after a single intraperitoneal (i.p.) injection of MS41 (50 mg/kg). Error bars represent means ± SD from three mice per time point. (B) Schematic of the MV4;11 xenotransplantation model and treatment workflow. (C) Kaplan-Meier survival curves of mice treated with vehicle (n = 11) and MS41 (n = 12, 50 mg/kg, once daily, i.p.) in the xenograft model. Log-rank test was used. (D) Quantification of MV4;11 cells (human CD45+) in the peripheral blood collected at the indicated time points from xenografted mice. Student’s t test, *P < 0.05, ***P < 0.001; ns, not significant. (E and F) Quantification of mean bioluminescence signals (E) and representative bioluminescence images (F) of xenografted mice at the indicated time points. Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant. (G and H) H&E (G) and Ki-67 immunohistochemistry (H) staining of bone marrow, liver, and spleen of moribund vehicle or MS41-treated xenografted mice. Scale bars, 100 μm. (I) Immunoblots for ENL and turboGFP of vehicle or MS41-treated bone marrow collected from moribund mice.