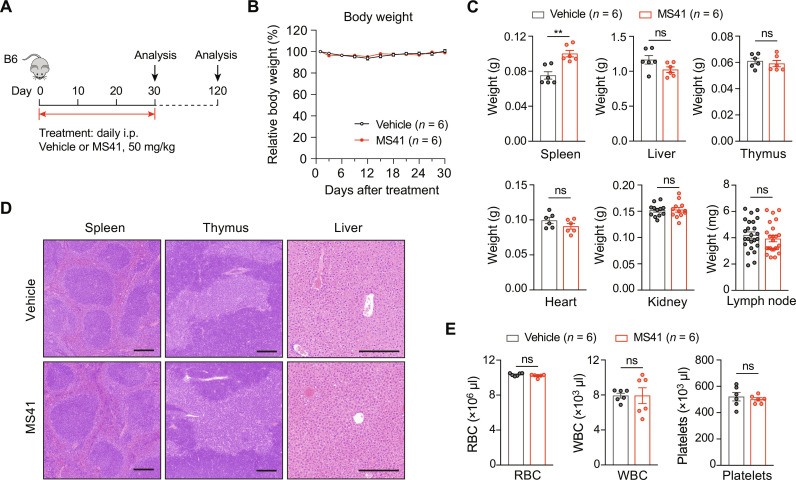
Fig. 7. MS41 exhibits no in vivo toxicity.
(A) Schematic of MS41 treatment workflow in C57BL/6 mice. (B) Quantification of relative body weight over 30 days of vehicle or MS41 (50 mg/kg, once daily, i.p.) treatment. Mouse body weight was measured every 3 days. (C) Weight quantification of spleen, liver, thymus, heart, kidney, and lymph node collected from mice at the end of 30 days treatment with vehicle and MS41. (D) H&E staining of spleen, thymus, and liver from mice as described in (C). Scale bars, 250 μm. (E) Complete blood count analyses of WBCs, RBCs, and platelets of peripheral blood collected from mice as described in (C). Error bars represent means ± SEM (n = 6). Unpaired two-tailed t test was used for calculating P values in (C) and (E). **P < 0.01; ns, not significant.