Abstract
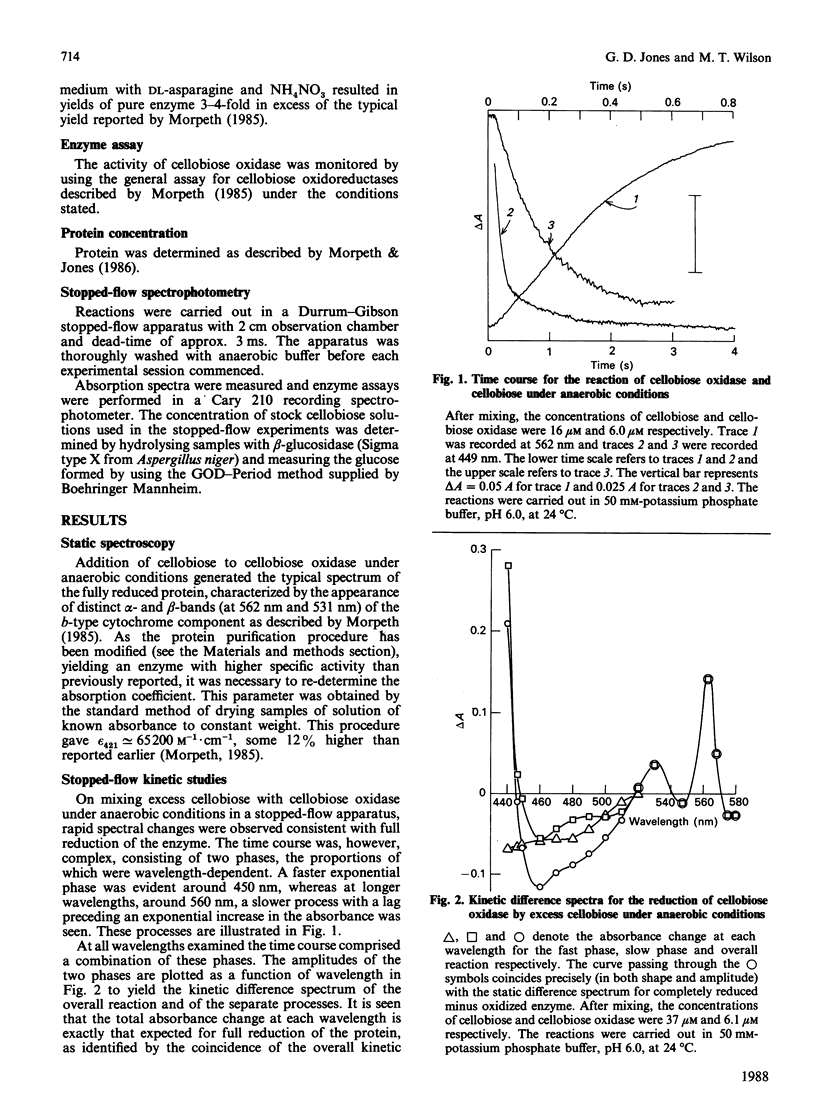
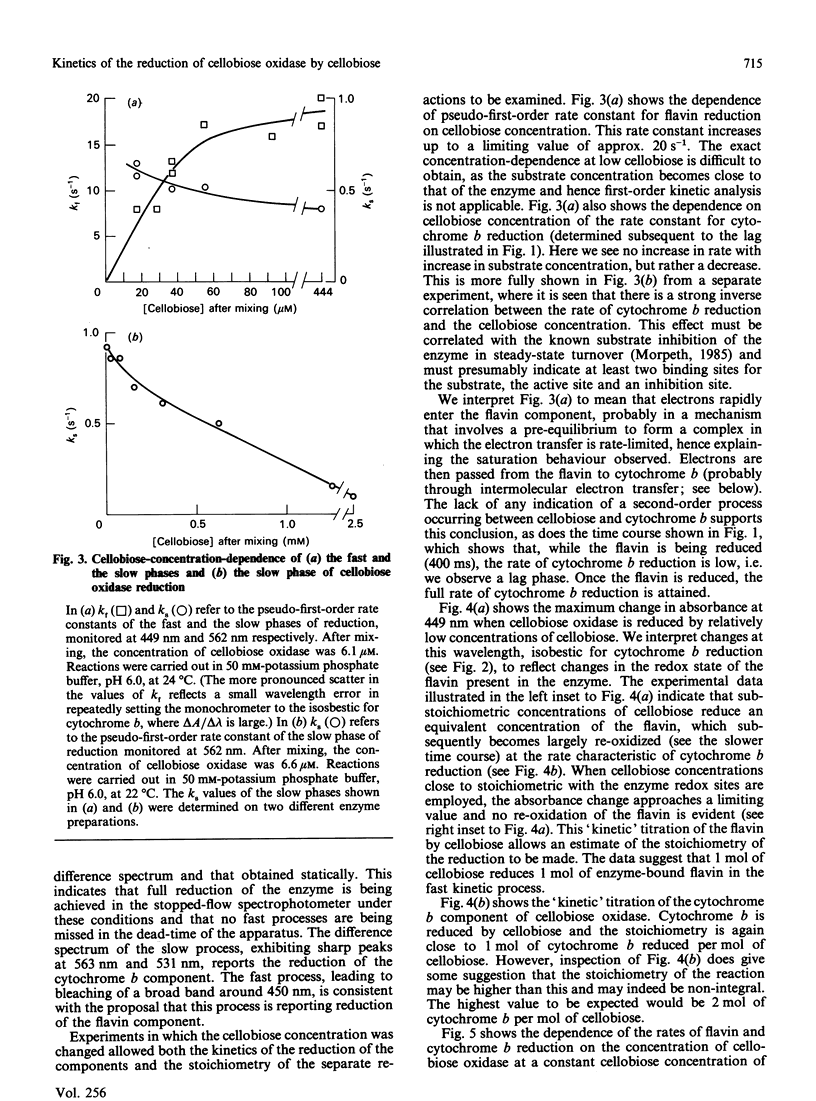
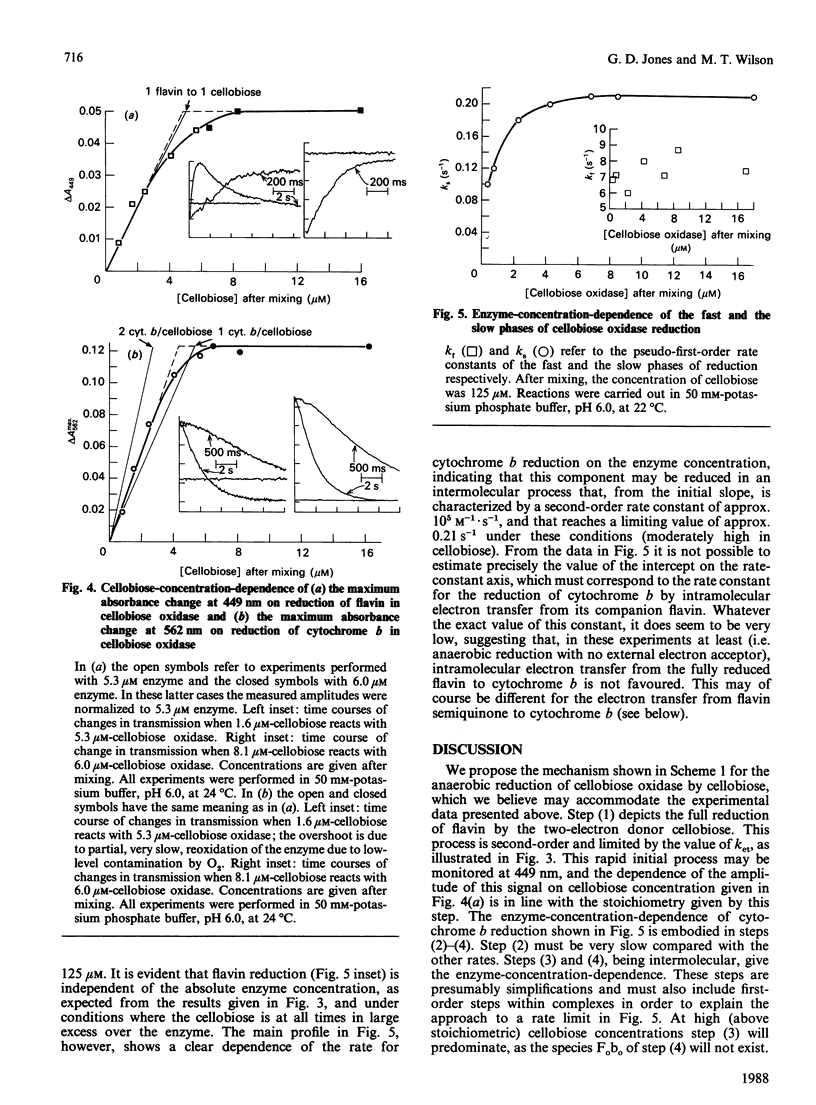
The reactions between cellobiose and cellobiose oxidase were investigated by stopped-flow spectrophotometry. Under anaerobic conditions rapid reduction of the associated flavin is followed by slower reduction of cytochrome b. The kinetic difference spectra are reported. The rate of flavin reduction depends on the cellobiose concentration (with an apparent second-order rate constant of approx. 10(5) M-1.s-1) but reaches a rate limit of approx. 20 s-1. In contrast, the rate of cytochrome b reduction decreases at high cellobiose concentrations. Kinetic titrations of the flavin and cytochrome b moieties yield the stoichiometries of the separate reactions, i.e. the number of moles of cellobiose needed to fully reduce 1 mole of each redox component. The rate constant for cytochrome b reduction, unlike that for flavin reduction, increased with enzyme concentration, prompting the conclusion that any given cytochrome b centre is reduced preferentially by flavin groups in different molecules rather than by its partner flavin within the same monomer. These data are discussed in the context of a scheme that rationalizes them and accounts for the overall stoichiometry in which three two-electron donors (cellobiose molecules) reduce two three-electron acceptors (the flavin-cytochrome b of cellobiose oxidase).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ayers S. B., Eriksson K. E. Cellobiose oxidase, purification and partial characterization of a hemoprotein from Sporotrichum pulverulentum. Eur J Biochem. 1978 Sep 15;90(1):171–181. doi: 10.1111/j.1432-1033.1978.tb12588.x. [DOI] [PubMed] [Google Scholar]
- Capeillère-Blandin C., Bray R. C., Iwatsubo M., Labeyrie F. Flavocytochrome b2: kinetic studies by absorbance and electron-paramagnetic-resonance spectroscopy of electron distribution among prosthetic groups. Eur J Biochem. 1975 Jun;54(2):549–566. doi: 10.1111/j.1432-1033.1975.tb04168.x. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Tien M., Kersten P. J., Mozuch M. D., Kalyanaraman B. Ligninase of Phanerochaete chrysosporium. Mechanism of its degradation of the non-phenolic arylglycerol beta-aryl ether substructure of lignin. Biochem J. 1986 May 15;236(1):279–287. doi: 10.1042/bj2360279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist K., Kristersson P. Exhaustive laccase-catalysed oxidation of a lignin model compound (vanillyl glycol) produces methanol and polymeric quinoid products. Biochem J. 1985 Jul 1;229(1):277–279. doi: 10.1042/bj2290277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpeth F. F., Jones G. D. Resolution, purification and some properties of the multiple forms of cellobiose quinone dehydrogenase from the white-rot fungus Sporotrichum pulverulentum. Biochem J. 1986 May 15;236(1):221–226. doi: 10.1042/bj2360221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpeth F. F. Some properties of cellobiose oxidase from the white-rot fungus Sporotrichum pulverulentum. Biochem J. 1985 Jun 15;228(3):557–564. doi: 10.1042/bj2280557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Westermark U., Eriksson K. E. Purification and properties of cellobiose: quinone oxidoreductase from Sporotrichum pulverulentum. Acta Chem Scand B. 1975;29(4):419–424. [PubMed] [Google Scholar]


