Abstract
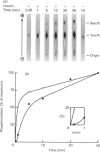
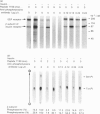
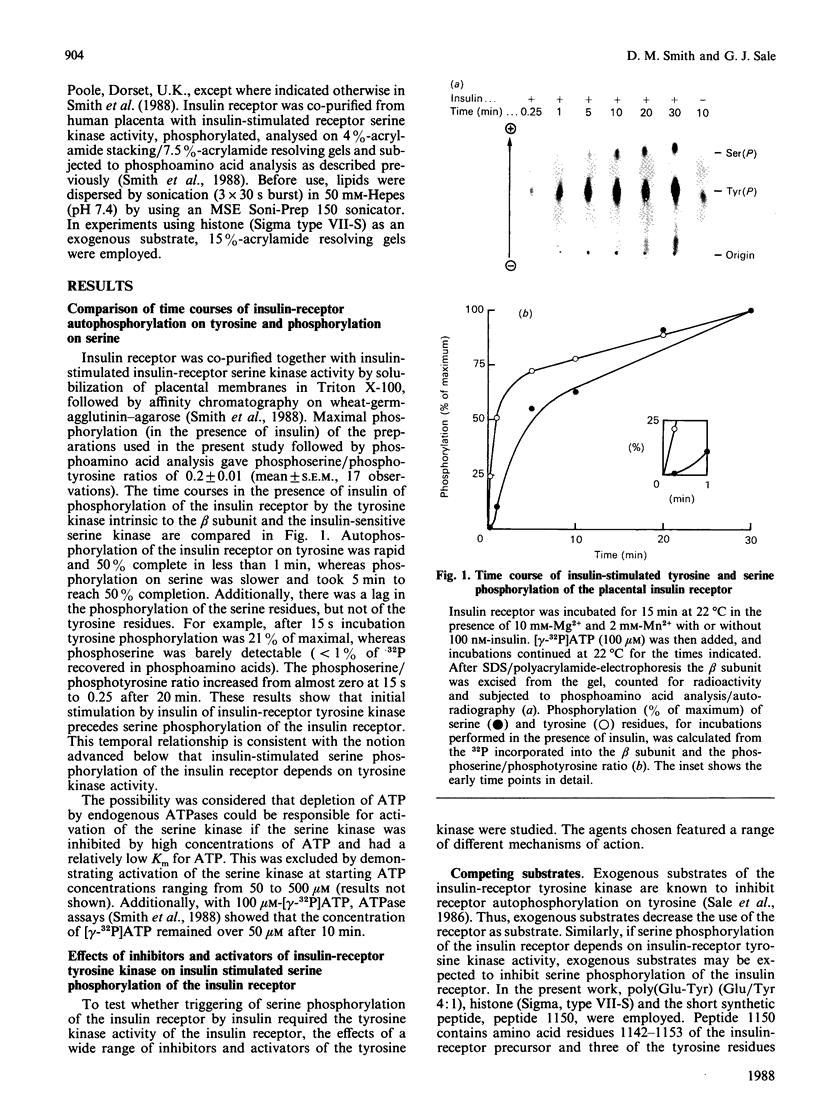
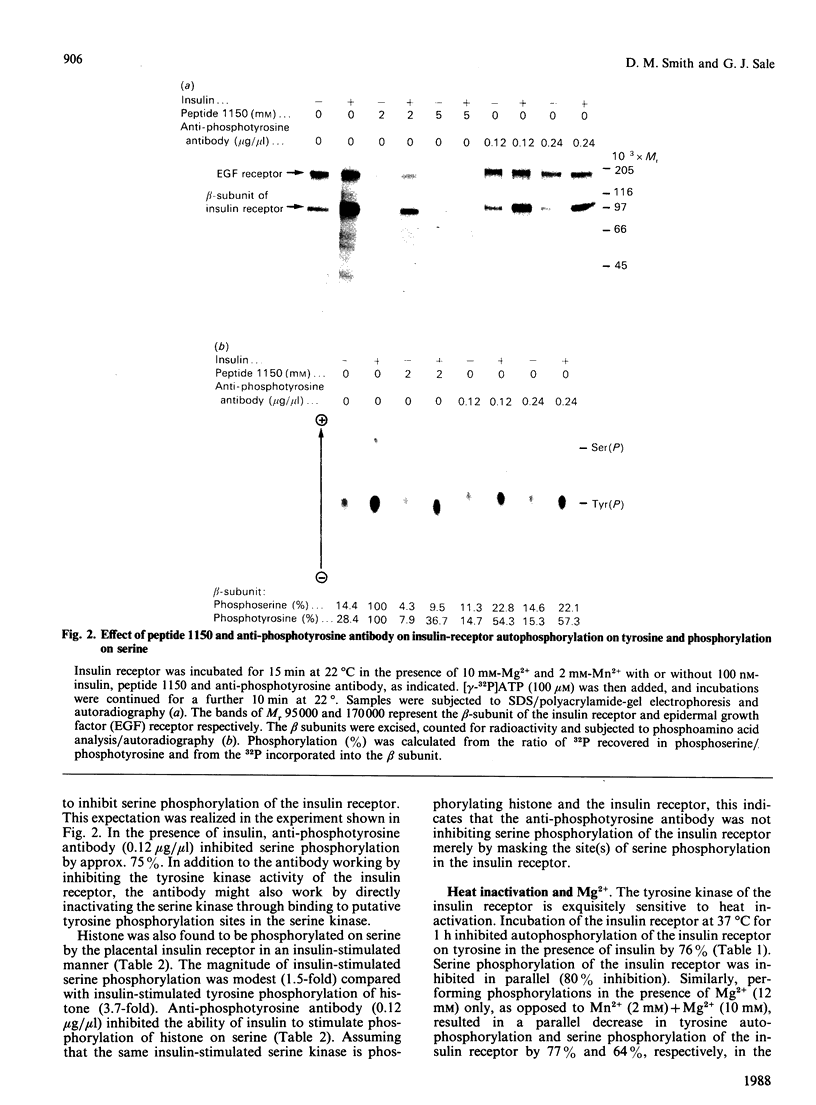
Insulin receptor was co-purified from human placenta together with insulin-stimulated kinase activity that phosphorylates the insulin receptor on serine residues. By using this 'in vitro' system, the mechanism of activation of the serine kinase by insulin was explored. Peptide 1150, histone, poly(Glu-Tyr), eliminating Mn2+ (Mg2+ only), treatment at 37 degrees C (1 h), N-ethylmaleimide, phosphate, beta-glycerol phosphate and anti-phosphotyrosine antibody all inhibited insulin-receptor tyrosine kinase activity and the ability of insulin to stimulate phosphorylation of the insulin receptor on serine. Additionally, direct stimulation of the receptor tyrosine kinase by vanadate increased serine phosphorylation of the insulin receptor. Insulin-stimulated tyrosine phosphorylation preceded insulin-stimulated serine phosphorylation of the insulin receptor. The activity of the insulin-sensitive receptor serine kinase was not augmented by cyclic AMP, cyclic GMP, Ca2+, Ca2+ + calmodulin, Ca2+ + phosphatidylserine + diolein or spermine, or inhibited appreciably by heparin. Additionally, the serine kinase phosphorylated casein or phosvitin poorly and was active with Mn2+. This indicates that it is distinct from Ca2+, Ca2+/phospholipid, Ca2+/calmodulin, cyclic AMP- and cyclic GMP-dependent protein kinases, casein kinases I and II and insulin-activated ribosomal S6 kinase. Taken together, these data indicate that a novel species of serine kinase catalyses the insulin-dependent phosphorylation of the insulin receptor and that activation of this receptor serine kinase by insulin requires an active insulin-receptor tyrosine kinase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollag G. E., Roth R. A., Beaudoin J., Mochly-Rosen D., Koshland D. E., Jr Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5822–5824. doi: 10.1073/pnas.83.16.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsey R. W., Edgell N. J., Hopkirk T. J., Denton R. M. Studies on insulin-stimulated phosphorylation of acetyl-CoA carboxylase, ATP citrate lyase and other proteins in rat epididymal adipose tissue. Evidence for activation of a cyclic AMP-independent protein kinase. Biochem J. 1984 Mar 15;218(3):733–743. doi: 10.1042/bj2180733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Cobb M. H. An insulin-stimulated ribosomal protein S6 kinase in 3T3-L1 cells. J Biol Chem. 1986 Oct 5;261(28):12994–12999. [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Brownsey R. W., Belsham G. J. A partial view of the mechanism of insulin action. Diabetologia. 1981 Oct;21(4):347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Maller J. L. Purification and characterization of a protein kinase from Xenopus eggs highly specific for ribosomal protein S6. J Biol Chem. 1986 Jan 5;261(1):350–355. [PubMed] [Google Scholar]
- Gazzano H., Kowalski A., Fehlmann M., Van Obberghen E. Two different protein kinase activities are associated with the insulin receptor. Biochem J. 1983 Dec 15;216(3):575–582. doi: 10.1042/bj2160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- King M. J., Sale G. J. Insulin-receptor phosphotyrosyl-protein phosphatases. Biochem J. 1988 Dec 15;256(3):893–902. doi: 10.1042/bj2560893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. Characterization of the phosphorylated form of the insulin-stimulated cyclic AMP phosphodiesterase from rat liver plasma membranes. Biochem J. 1981 Jun 1;195(3):653–660. doi: 10.1042/bj1950653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato J. M., Kelly K. L., Abler A., Jarett L. Identification of a novel insulin-sensitive glycophospholipid from H35 hepatoma cells. J Biol Chem. 1987 Feb 15;262(5):2131–2137. [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Acute insulin action requires insulin receptor kinase activity: introduction of an inhibitory monoclonal antibody into mammalian cells blocks the rapid effects of insulin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):41–45. doi: 10.1073/pnas.84.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. J., Kuenzel E. A., Casnellie J. E., Krebs E. G. A comparison of the insulin- and epidermal growth factor-stimulated protein kinases from human placenta. J Biol Chem. 1984 Aug 10;259(15):9913–9921. [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale E. M., Denton R. M. Beta-adrenergic agents increase the phosphorylation of phosphofructokinase in isolated rat epididymal white adipose tissue. Biochem J. 1985 Dec 15;232(3):905–910. doi: 10.1042/bj2320905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale G. J., Fujita-Yamaguchi Y., Kahn C. R. Characterization of phosphatidylinositol kinase activity associated with the insulin receptor. Eur J Biochem. 1986 Mar 3;155(2):345–351. doi: 10.1111/j.1432-1033.1986.tb09497.x. [DOI] [PubMed] [Google Scholar]
- Sale G. J., Randle P. J. Occupancy of phosphorylation sites in pyruvate dehydrogenase phosphate complex in rat heart in vivo. Relation to proportion of inactive complex and rate of re-activation by phosphatase. Biochem J. 1982 Aug 15;206(2):221–229. doi: 10.1042/bj2060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale G. J. Recent progress in our understanding of the mechanism of action of insulin. Int J Biochem. 1988;20(9):897–908. doi: 10.1016/0020-711x(88)90173-5. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Sherline P., Fox J. A. Insulin-stimulated diacylglycerol production results from the hydrolysis of a novel phosphatidylinositol glycan. J Biol Chem. 1987 Jan 25;262(3):1116–1121. [PubMed] [Google Scholar]
- Smith D. M., King M. J., Sale G. J. Two systems in vitro that show insulin-stimulated serine kinase activity towards the insulin receptor. Biochem J. 1988 Mar 1;250(2):509–519. doi: 10.1042/bj2500509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach D. H., Nemenoff R. A., Blackshear P. J. Protein phosphorylation and protein kinase activities in BC3H-1 myocytes. Differences between the effects of insulin and phorbol esters. J Biol Chem. 1986 Sep 25;261(27):12750–12753. [PubMed] [Google Scholar]
- Stadtmauer L., Rosen O. M. Increasing the cAMP content of IM-9 cells alters the phosphorylation state and protein kinase activity of the insulin receptor. J Biol Chem. 1986 Mar 5;261(7):3402–3407. [PubMed] [Google Scholar]
- Tabarini D., Heinrich J., Rosen O. M. Activation of S6 kinase activity in 3T3-L1 cells by insulin and phorbol ester. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4369–4373. doi: 10.1073/pnas.82.13.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- White M. F., Shoelson S. E., Keutmann H., Kahn C. R. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988 Feb 25;263(6):2969–2980. [PubMed] [Google Scholar]
- Yu K. T., Khalaf N., Czech M. P. Insulin stimulates a membrane-bound serine kinase that may be phosphorylated on tyrosine. Proc Natl Acad Sci U S A. 1987 Jun;84(12):3972–3976. doi: 10.1073/pnas.84.12.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]