Abstract
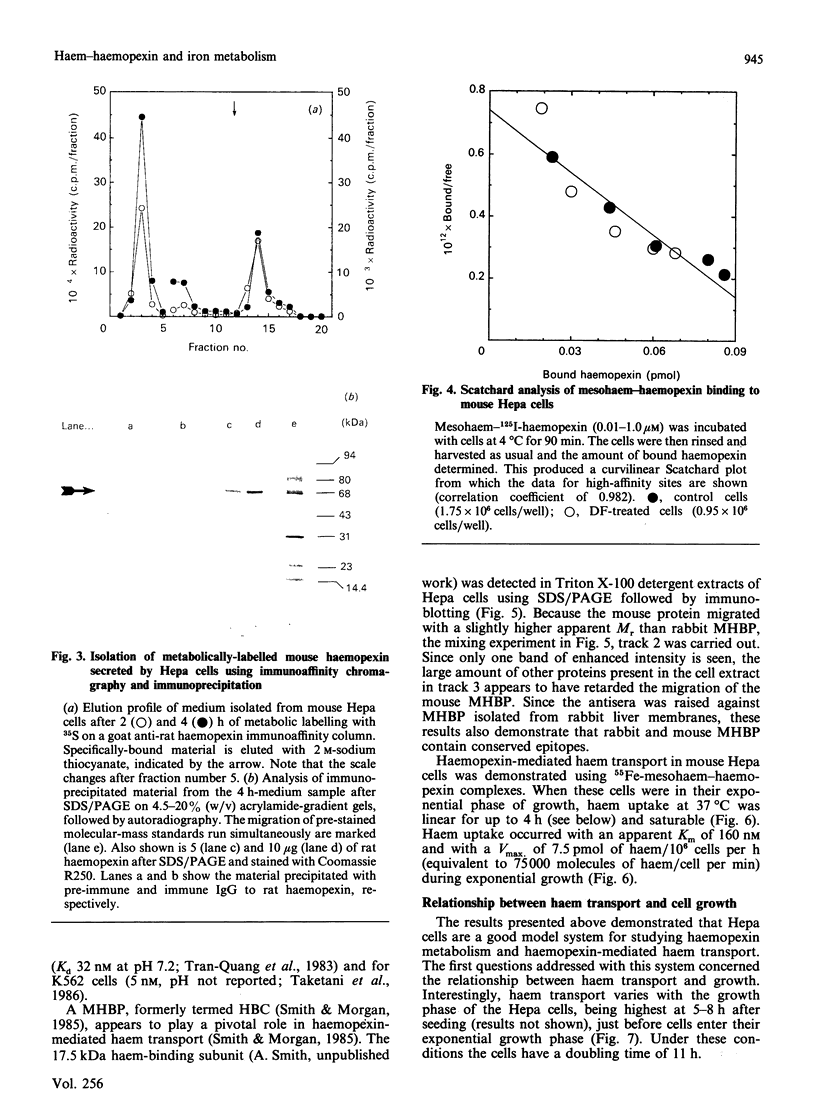
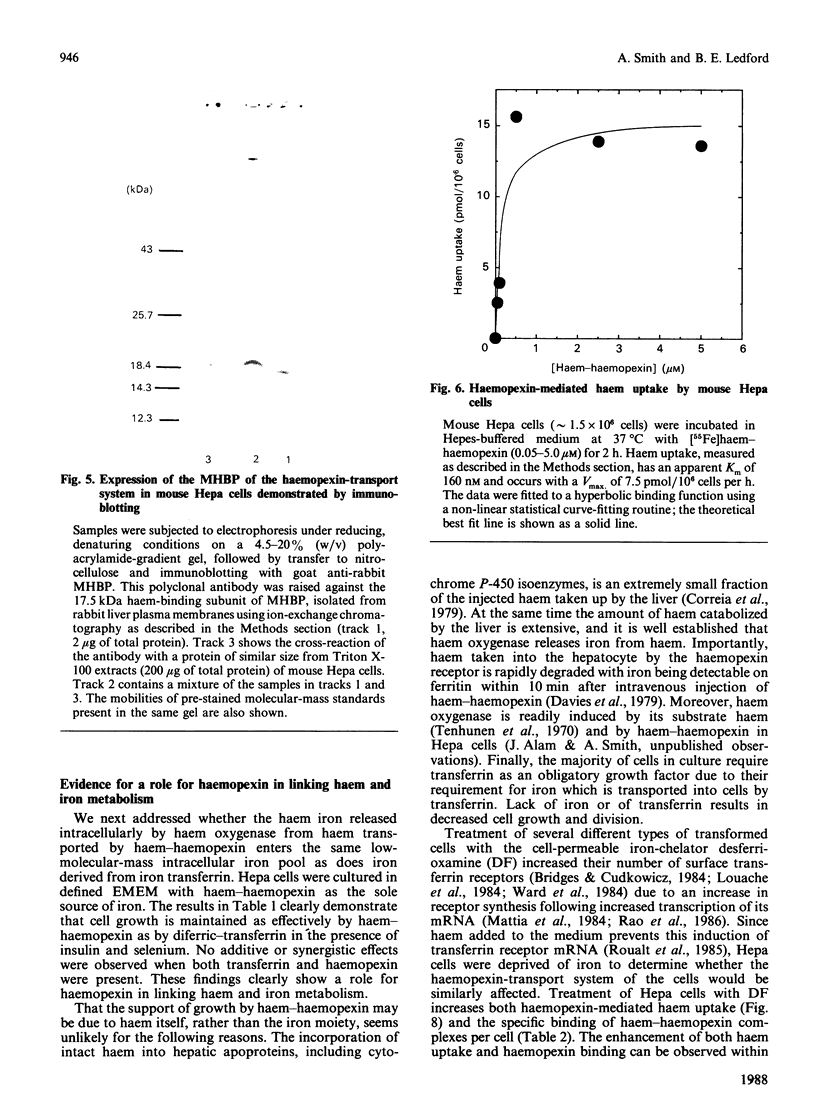
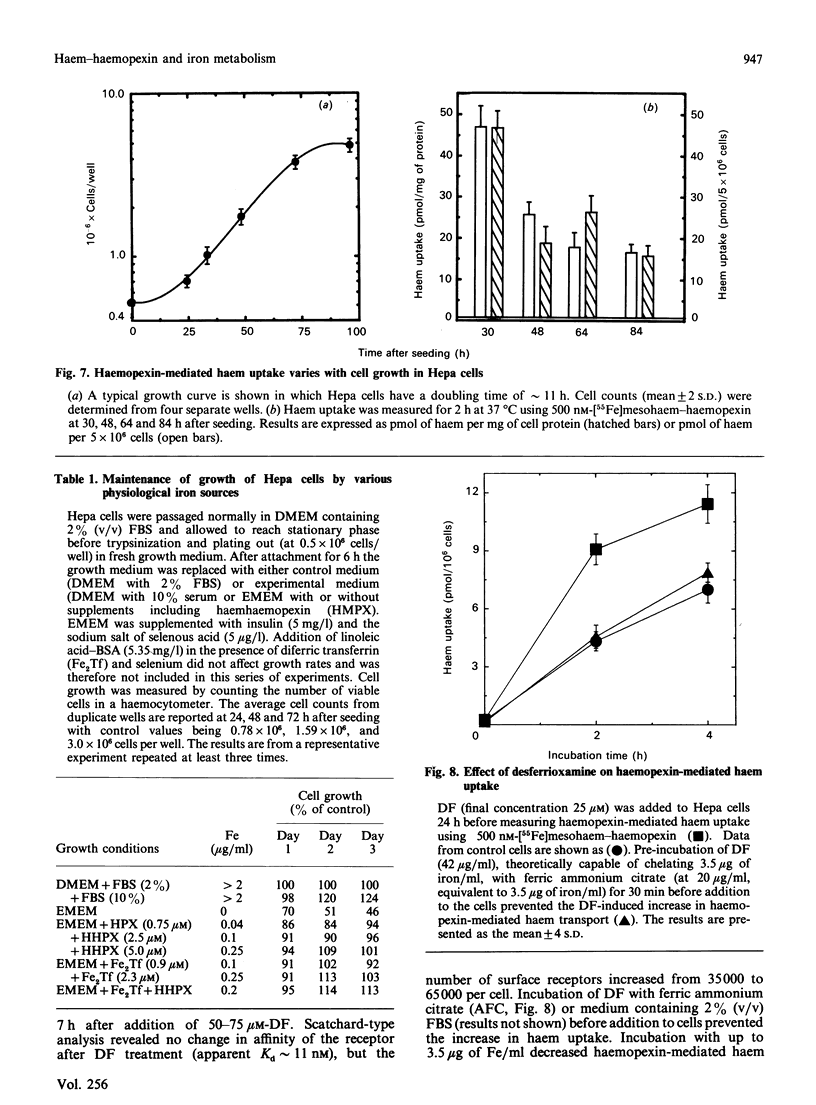
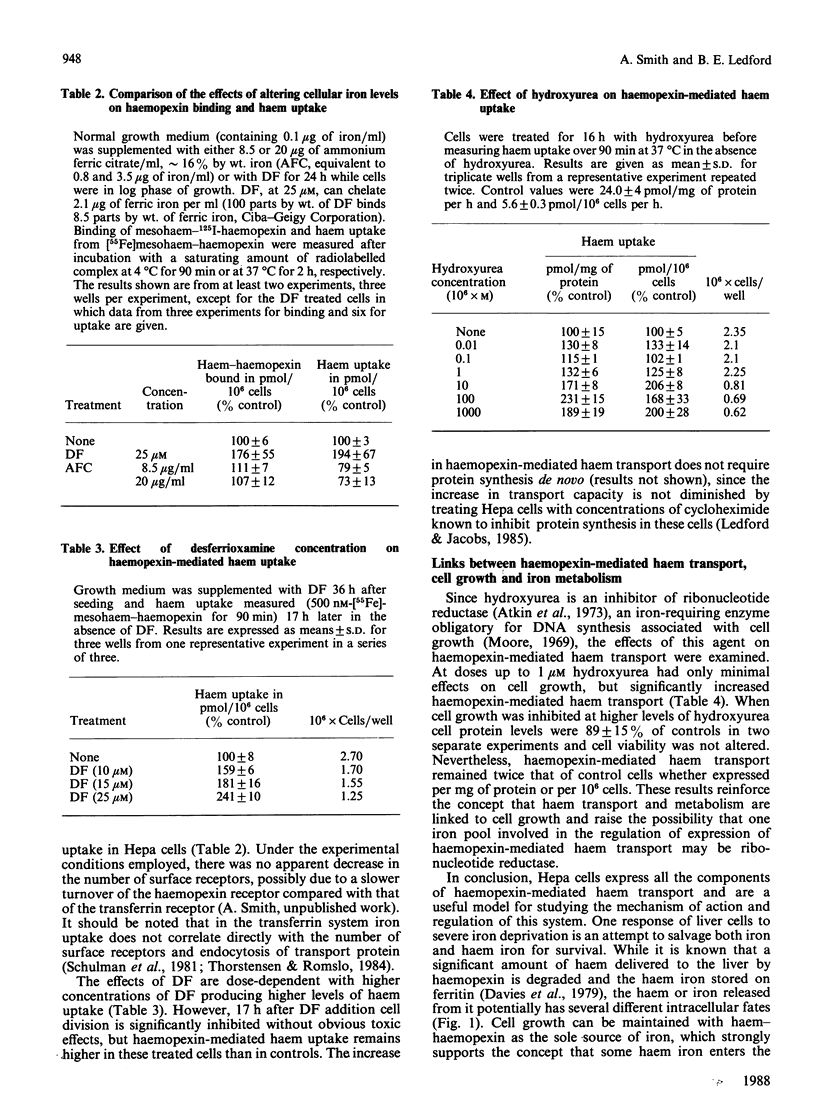
Minimal deviation hepatoma (Hepa) cells, from the mouse hepatoma B7756, synthesize and secrete haemopexin and express both the haemopexin receptor and the membrane haem-binding protein (MHBP) associated with the receptor, making this cell line the first available for detailed study of both haemopexin metabolism and hepatic transport. The 17.5 kDa MHBP was detected in Triton X-100 extracts of Hepa cells by immunoblotting with goat anti-rabbit MHBP. Scatchard-type analysis of haem-125I-haemopexin binding at 4 degrees C revealed 35,000 receptors per cell of high affinity (Kd 17 nM). Haemopexin-mediated haem transport at 37 degrees C is saturable, having an apparent Km of 160 nM and a Vmax. of 7.5 pmol of haem/10(6) cells per h during exponential growth. Haem-transport capacity is highest in the period just before the cells enter their exponential phase of growth and slowest in stationary phase. Interestingly, haem-haemopexin serves as effectively as iron-transferrin as the sole source of iron for cell growth by Hepa cells. Furthermore, depriving Hepa cells of iron by treatment with desferrioxamine (DF) increases the number of cell-surface haemopexin receptors to 65,000 per cell and consequently increases haemopexin-mediated haem transport. The effects of DF do not appear to require protein synthesis since they are not prevented by cycloheximide. Treatment of Hepa cells with hydroxyurea, an inhibitor of the iron-requiring enzyme ribonucleotide reductase that is obligatory for DNA synthesis, enhanced haemopexin-mediated haem transport. Thus, these studies provide the first evidence for regulation of haem transport by the iron status of cells and suggest a linkage between haemopexin, iron homeostasis and cell growth.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin C. L., Thelander L., Reichard P., Lang G. Iron and free radical in ribonucleotide reductase. Exchange of iron and Mössbauer spectroscopy of the protein B2 subunit of the Escherichia coli enzyme. J Biol Chem. 1973 Nov 10;248(21):7464–7472. [PubMed] [Google Scholar]
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Baumann H., Held W. A., Berger F. G. The acute phase response of mouse liver. Genetic analysis of the major acute phase reactants. J Biol Chem. 1984 Jan 10;259(1):566–573. [PubMed] [Google Scholar]
- Baumann H., Onorato V., Gauldie J., Jahreis G. P. Distinct sets of acute phase plasma proteins are stimulated by separate human hepatocyte-stimulating factors and monokines in rat hepatoma cells. J Biol Chem. 1987 Jul 15;262(20):9756–9768. [PubMed] [Google Scholar]
- Bernhard H. P., Darlington G. J., Ruddle F. H. Expression of liver phenotypes in cultured mouse hepatoma cells: synthesis and secretion of serum albumin. Dev Biol. 1973 Nov;35(1):83–96. doi: 10.1016/0012-1606(73)90008-0. [DOI] [PubMed] [Google Scholar]
- Braun S. R., Weiss F. R., Keller A. I., Ciccone J. R., Preuss H. G. Evaluation of the renal toxicity of heme proteins and their derivatives: a role in the genesis of acute tubule necrosis. J Exp Med. 1970 Mar 1;131(3):443–460. doi: 10.1084/jem.131.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges K. R., Cudkowicz A. Effect of iron chelators on the transferrin receptor in K562 cells. J Biol Chem. 1984 Nov 10;259(21):12970–12977. [PubMed] [Google Scholar]
- Brown S. B., Lantzke I. R. Solution structures of ferrihaem in some dipolar aprotic solvents and their binary aqueous mixtures. Biochem J. 1969 Nov;115(2):279–285. doi: 10.1042/bj1150279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel N., Gross J. Hemopexin metabolism in mice with transplantable tumors. Isr J Med Sci. 1977 Dec;13(12):1182–1190. [PubMed] [Google Scholar]
- Correia M. A., Farrell G. C., Schmid R., Ortiz de Montellano P. R., Yost G. S., Mico B. A. Incorporation of exogenous heme into hepatic cytochrome P-450 in vivo. J Biol Chem. 1979 Jan 10;254(1):15–17. [PubMed] [Google Scholar]
- Davies D. M., Smith A., Muller-Eberhard U., Morgan W. T. Hepatic subcellular metabolism of heme from heme-hemopexin: incorporation of iron into ferritin. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1504–1511. doi: 10.1016/0006-291x(79)91235-x. [DOI] [PubMed] [Google Scholar]
- Glass J., Nunez M. T., Robinson S. H. Transferrin-binding and iron-binding proteins of rabbit reticulocyte plasma membranes. Three distinct moieties. Biochim Biophys Acta. 1980 May 23;598(2):293–304. doi: 10.1016/0005-2736(80)90007-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Smith A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem J. 1988 Dec 15;256(3):861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa Y., Oshiro S., Kino K., Tsunoo H., Nakajima H. Catabolism of globin-haptoglobin in liver cells after intravenous administration of hemoglobin-haptoglobin to rats. J Biol Chem. 1981 Dec 10;256(23):12322–12328. [PubMed] [Google Scholar]
- Hrkal Z., Muller-Eberhard U. Partial characterization of the heme-binding serum glycoproteins rabbit and human hemopexin. Biochemistry. 1971 May 11;10(10):1746–1750. doi: 10.1021/bi00786a002. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Zabriskie J. B. Purification and partial characterization of the nephritis strain-associated protein from Streptococcus pyogenes, group A. J Exp Med. 1986 Mar 1;163(3):697–712. doi: 10.1084/jem.163.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino K., Tsunoo H., Higa Y., Takami M., Nakajima H. Kinetic aspects of hemoglobin.haptoglobin-receptor interaction in rat liver plasma membranes, isolated liver cells, and liver cells in primary culture. J Biol Chem. 1982 May 10;257(9):4828–4833. [PubMed] [Google Scholar]
- Kochan I. The role of iron in bacterial infections, with special consideration of host-tubercle bacillus interaction. Curr Top Microbiol Immunol. 1973;60:1–30. doi: 10.1007/978-3-642-65502-9_1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledford B. E., Davis D. F. Kinetics of serum protein secretion by cultured hepatoma cells. Evidence for multiple secretory pathways. J Biol Chem. 1983 Mar 10;258(5):3304–3308. [PubMed] [Google Scholar]
- Ledford B. E., Jacobs D. F. Translation kinetics in cultured mouse hepatoma cells. Regulation of albumin synthesis by amino acids. Eur J Biochem. 1985 Nov 4;152(3):611–618. doi: 10.1111/j.1432-1033.1985.tb09239.x. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Zhai S., Lawler J., Palek J. Hemin-mediated dissociation of erythrocyte membrane skeletal proteins. J Biol Chem. 1985 Oct 5;260(22):12234–12239. [PubMed] [Google Scholar]
- Louache F., Testa U., Pelicci P., Thomopoulos P., Titeux M., Rochant H. Regulation of transferrin receptors in human hematopoietic cell lines. J Biol Chem. 1984 Sep 25;259(18):11576–11582. [PubMed] [Google Scholar]
- Majuri R., Gräsbeck R. Isolation of the haemopexin-haem receptor from pig liver cells. FEBS Lett. 1986 Apr 7;199(1):80–84. doi: 10.1016/0014-5793(86)81227-3. [DOI] [PubMed] [Google Scholar]
- Mattia E., Rao K., Shapiro D. S., Sussman H. H., Klausner R. D. Biosynthetic regulation of the human transferrin receptor by desferrioxamine in K562 cells. J Biol Chem. 1984 Mar 10;259(5):2689–2692. [PubMed] [Google Scholar]
- McCracken A. A., Emmett M., Crowle A. J., Brown J. L. Studies on the secretion of serum proteins from rat hepatoma cells. Hepatology. 1984 Jul-Aug;4(4):715–721. doi: 10.1002/hep.1840040426. [DOI] [PubMed] [Google Scholar]
- Merriman C. R., Upchurch H. F., Kampschmidt R. F. Effects of leukocytic endogenous mediator on hemopexin, transferr, and liver catalase. Proc Soc Exp Biol Med. 1978 Apr;157(4):669–671. doi: 10.3181/00379727-157-40118. [DOI] [PubMed] [Google Scholar]
- Moore E. C. The effects of ferrous ion and dithioerythritol on inhibition by hydroxyruea of ribonucleotide reductase. Cancer Res. 1969 Feb;29(2):291–295. [PubMed] [Google Scholar]
- Morgan W. T., Smith A. Domain structure of rabbit hemopexin. Isolation and characterization of a heme-binding glycopeptide. J Biol Chem. 1984 Oct 10;259(19):12001–12006. [PubMed] [Google Scholar]
- Müller R. M., Taguchi H., Shibahara S. Nucleotide sequence and organization of the rat heme oxygenase gene. J Biol Chem. 1987 May 15;262(14):6795–6802. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- OWEN J. A., SMITH R., PADANYI R., MARTIN J. SERUM HAPTOGLOBIN IN DISEASE. Clin Sci. 1964 Feb;26:1–6. [PubMed] [Google Scholar]
- Rao K., Harford J. B., Rouault T., McClelland A., Ruddle F. H., Klausner R. D. Transcriptional regulation by iron of the gene for the transferrin receptor. Mol Cell Biol. 1986 Jan;6(1):236–240. doi: 10.1128/mcb.6.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T., Rao K., Harford J., Mattia E., Klausner R. D. Hemin, chelatable iron, and the regulation of transferrin receptor biosynthesis. J Biol Chem. 1985 Nov 25;260(27):14862–14866. [PubMed] [Google Scholar]
- Schulman H. M., Wilczynska A., Ponka P. Transferrin and iron uptake by human lymphoblastoid and K-562 cells. Biochem Biophys Res Commun. 1981 Jun;100(4):1523–1530. doi: 10.1016/0006-291x(81)90691-4. [DOI] [PubMed] [Google Scholar]
- Seery V. L., Hathaway G., Eberhard U. M. Hemopexin of human and rabbit: molecular weight and extinction coefficient. Arch Biochem Biophys. 1972 May;150(1):269–272. doi: 10.1016/0003-9861(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Haem transport to the liver by haemopexin. Receptor-mediated uptake with recycling of the protein. Biochem J. 1979 Jul 15;182(1):47–54. doi: 10.1042/bj1820047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Hemopexin-mediated heme transport to the liver. Evidence for a heme-binding protein in liver plasma membranes. J Biol Chem. 1985 Jul 15;260(14):8325–8329. [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Hemopexin-mediated heme uptake by liver. Characterization of the interaction of heme-hemopexin with isolated rabbit liver plasma membranes. J Biol Chem. 1984 Oct 10;259(19):12049–12053. [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Hemopexin-mediated transport of heme into isolated rat hepatocytes. J Biol Chem. 1981 Nov 10;256(21):10902–10909. [PubMed] [Google Scholar]
- Smith A., Nuiry I., Morgan W. T. Proteolysis of histidine-rich glycoprotein in plasma and in patients undergoing thrombolytic therapy. Thromb Res. 1985 Dec 1;40(5):653–661. doi: 10.1016/0049-3848(85)90303-2. [DOI] [PubMed] [Google Scholar]
- Taketani S., Kohno H., Naitoh Y., Tokunaga R. Isolation of the hemopexin receptor from human placenta. J Biol Chem. 1987 Jun 25;262(18):8668–8671. [PubMed] [Google Scholar]
- Taketani S., Kohno H., Tokunaga R. Cell surface receptor for hemopexin in human leukemia HL60 cells. Specific binding, affinity labeling, and fate of heme. J Biol Chem. 1987 Apr 5;262(10):4639–4643. [PubMed] [Google Scholar]
- Taketani S., Kohno H., Tokunaga R. Receptor-mediated heme uptake from hemopexin by human erythroleukemia K562 cells. Biochem Int. 1986 Aug;13(2):307–312. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970 Mar;75(3):410–421. [PubMed] [Google Scholar]
- Thorbecke G. J., Liem H. H., Knight S., Cox K., Muller-Eberhard U. Sites of formation of the serum proteins transferrin and hemopexin. J Clin Invest. 1973 Mar;52(3):725–731. doi: 10.1172/JCI107234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstensen K., Romslo I. Uptake of iron from transferrin by isolated hepatocytes. Biochim Biophys Acta. 1984 Jun 19;804(2):200–208. doi: 10.1016/0167-4889(84)90150-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Quang N., Bernard N., Higa Y., Engler R. In vitro studies on some parameters of the binding of the rat hemopexin--heme complex with the hepatic membrane receptor. FEBS Lett. 1983 Aug 8;159(1-2):161–166. doi: 10.1016/0014-5793(83)80438-4. [DOI] [PubMed] [Google Scholar]
- Ward J. H., Jordan I., Kushner J. P., Kaplan J. Heme regulation of HeLa cell transferrin receptor number. J Biol Chem. 1984 Nov 10;259(21):13235–13240. [PubMed] [Google Scholar]