Abstract
The neuroblastoma-like cell line N2A and the pheochromocytoma-like cell line PC12 excrete about 20-25% of the intracellular fluorescent Ca2+ indicator fura-2 during 10 min of incubation at 37 degrees C. The drug probenecid, known to inhibit membrane systems for the transport of organic anions [Cunningham, Israili & Dayton (1981) Clin. Pharmacol. 6, 135-151], inhibited fura-2 excretion in both cell types. However, probenecid also had untoward effects on intracellular Ca2+ homeostasis in N2A and PC12 cells. We therefore tested the drug sulphinpyrazone, another known inhibitor of organic-anion transport systems. Sulphinpyrazone fully inhibited excretion of fura-2 at 250 microM, a concentration one order of magnitude lower than that of probenecid. At this concentration and for incubation times up to 20 min, sulphinpyrazone had no untoward effects on cell viability and metabolic functions. Fura-2 was also loaded into the cytoplasm of N2A cells by permeabilization of the plasma membrane with extracellular ATP. In this case as well, the dye was rapidly released from the cells and the efflux was blocked by sulphinpyrazone. These findings suggest that N2A and PC12 cells possess a membrane system for the transport of the free-acid form of fura-2. This transport system is probably responsible for the excretion of fura-2 from these cells. Incubation of N2A and PC12 cells with sulphinpyrazone may help overcome problems arising in the investigation of [Ca2+]i homeostasis in these cell types.
Full text
PDF
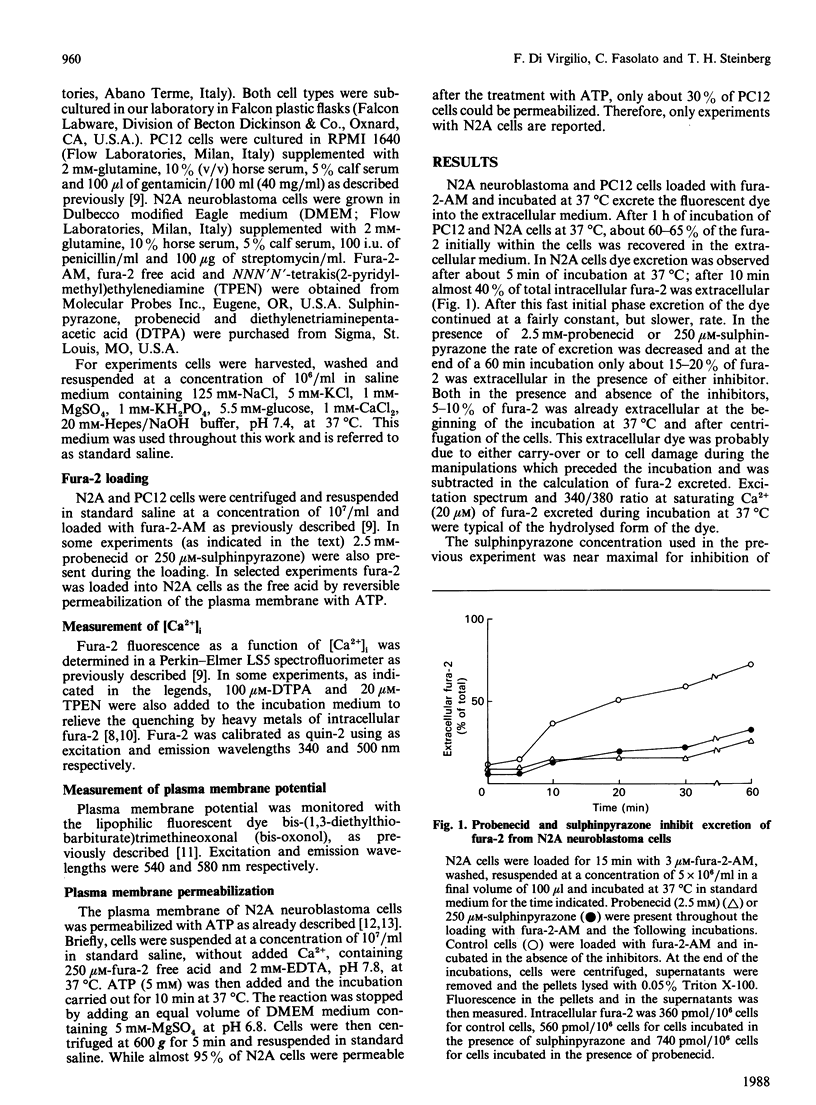
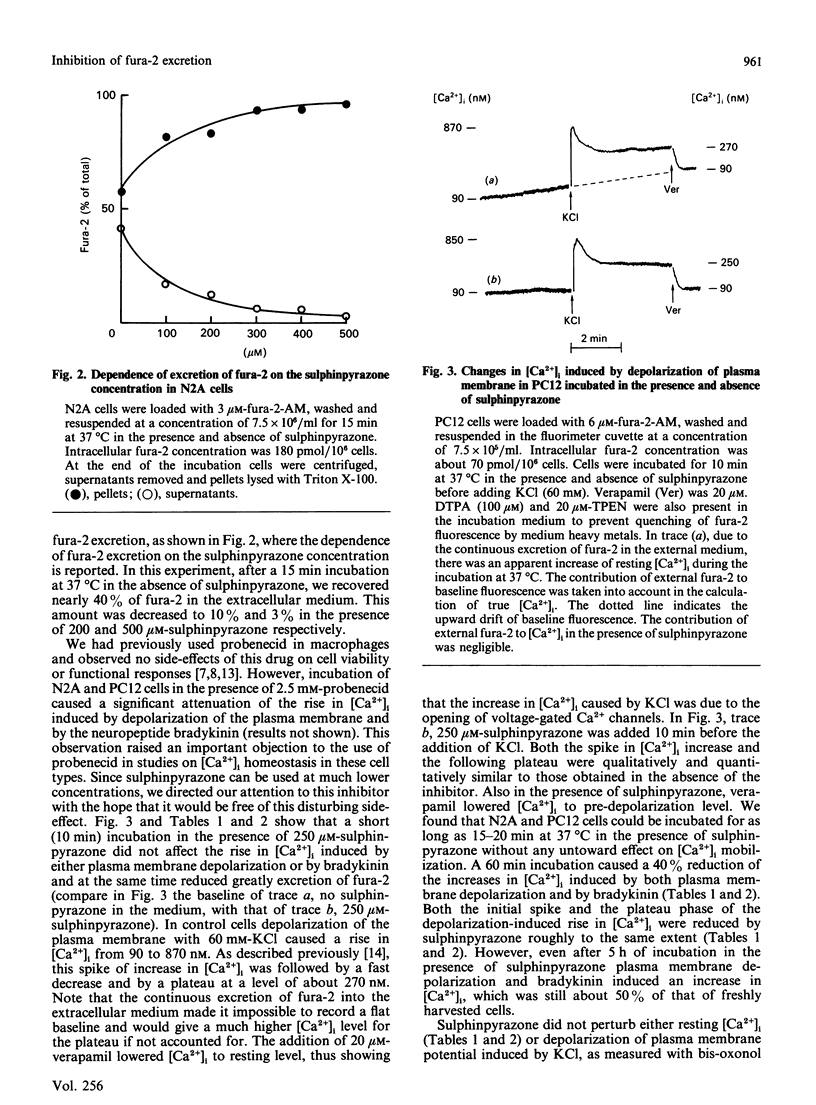
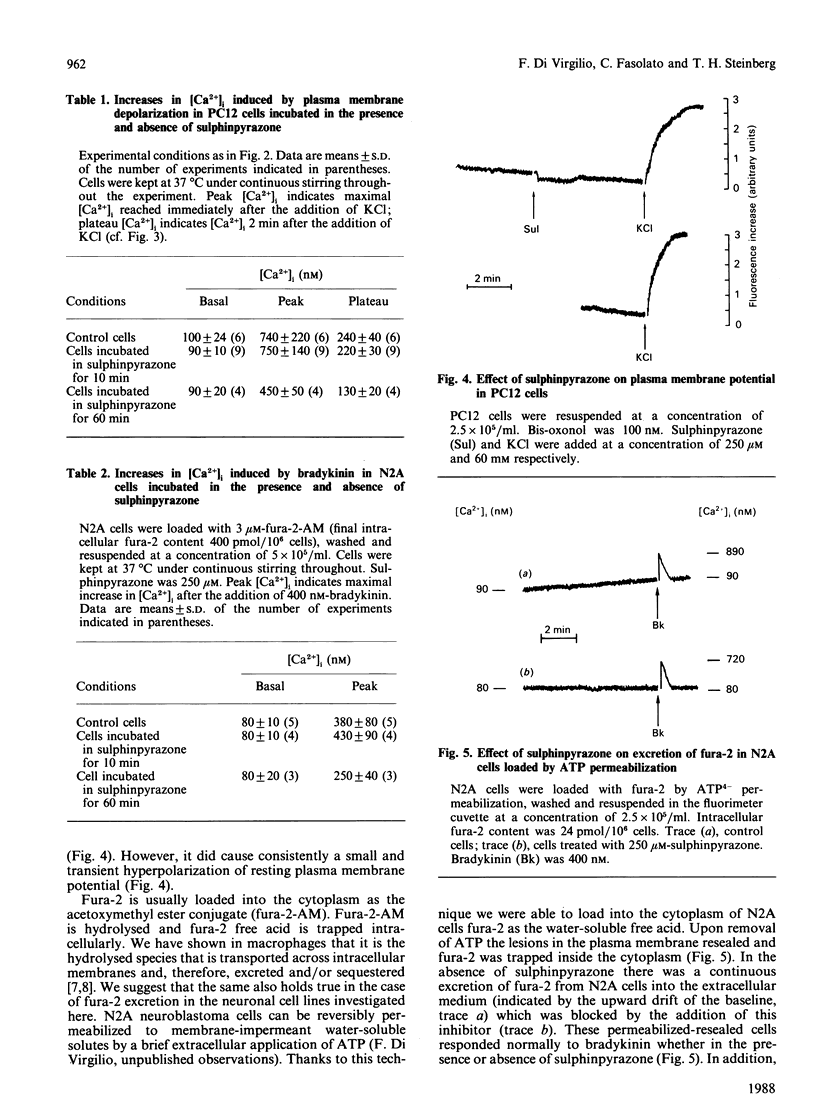

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Cunningham R. F., Israili Z. H., Dayton P. G. Clinical pharmacokinetics of probenecid. Clin Pharmacokinet. 1981 Mar-Apr;6(2):135–151. doi: 10.2165/00003088-198106020-00004. [DOI] [PubMed] [Google Scholar]
- De Togni P., Cabrini G., Di Virgilio F. Cyclic AMP inhibition of fMet-Leu-Phe-dependent metabolic responses in human neutrophils is not due to its effects on cytosolic Ca2+. Biochem J. 1984 Dec 1;224(2):629–635. doi: 10.1042/bj2240629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Meyer B. C., Greenberg S., Silverstein S. C. Fc receptor-mediated phagocytosis occurs in macrophages at exceedingly low cytosolic Ca2+ levels. J Cell Biol. 1988 Mar;106(3):657–666. doi: 10.1083/jcb.106.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Milani D., Leon A., Meldolesi J., Pozzan T. Voltage-dependent activation and inactivation of calcium channels in PC12 cells. Correlation with neurotransmitter release. J Biol Chem. 1987 Jul 5;262(19):9189–9195. [PubMed] [Google Scholar]
- Di Virgilio F., Steinberg T. H., Swanson J. A., Silverstein S. C. Fura-2 secretion and sequestration in macrophages. A blocker of organic anion transport reveals that these processes occur via a membrane transport system for organic anions. J Immunol. 1988 Feb 1;140(3):915–920. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Malgaroli A., Milani D., Meldolesi J., Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987 Nov;105(5):2145–2155. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Di Virgilio F., Vicentini L. M., Meldolesi J. Activation of muscarinic receptors in PC12 cells. Stimulation of Ca2+ influx and redistribution. Biochem J. 1986 Mar 15;234(3):547–553. doi: 10.1042/bj2340547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg T. H., Newman A. S., Swanson J. A., Silverstein S. C. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987 Jun 25;262(18):8884–8888. [PubMed] [Google Scholar]
- Treves S., Di Virgilio F., Cerundolo V., Zanovello P., Collavo D., Pozzan T. Calcium and inositolphosphates in the activation of T cell-mediated cytotoxicity. J Exp Med. 1987 Jul 1;166(1):33–42. doi: 10.1084/jem.166.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]


