Abstract
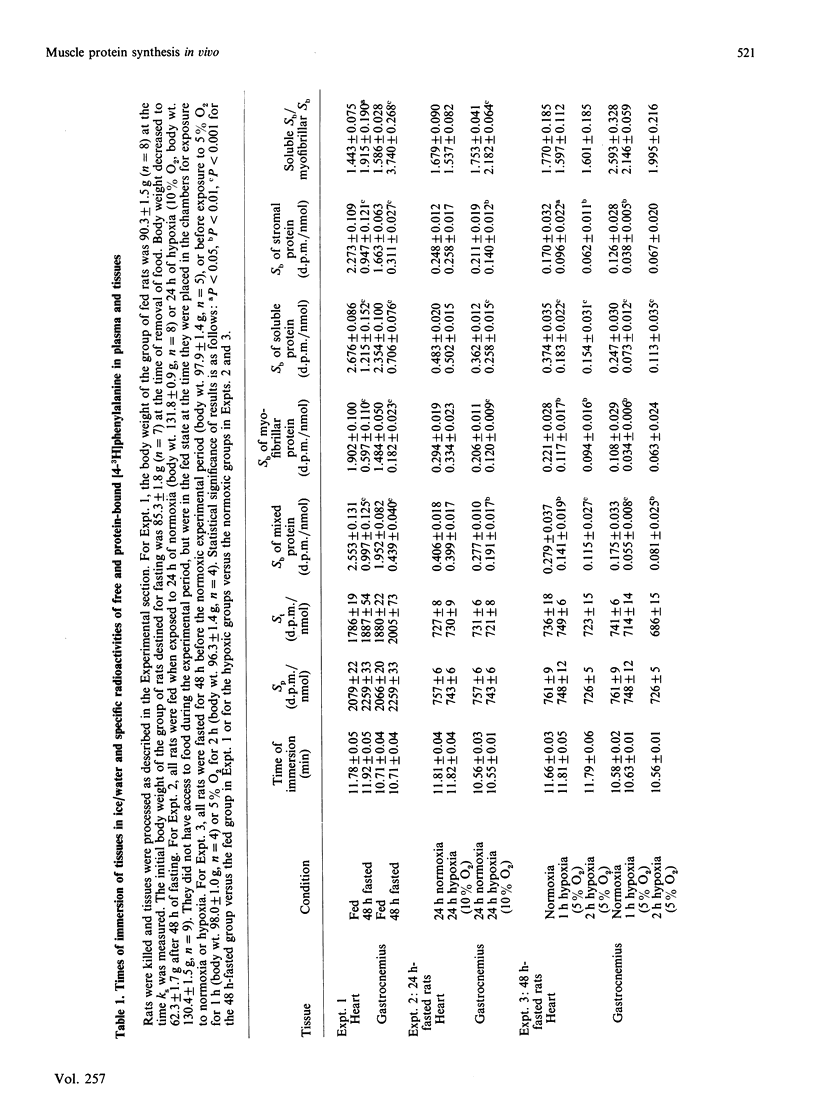
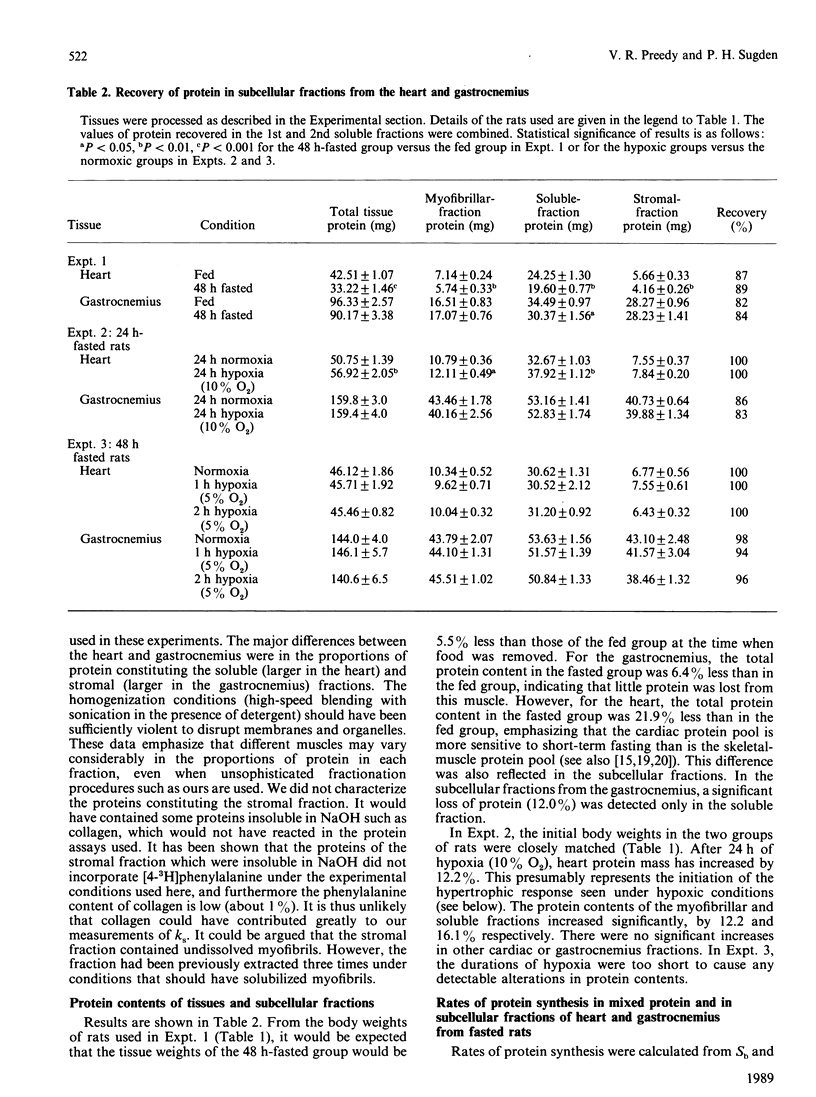
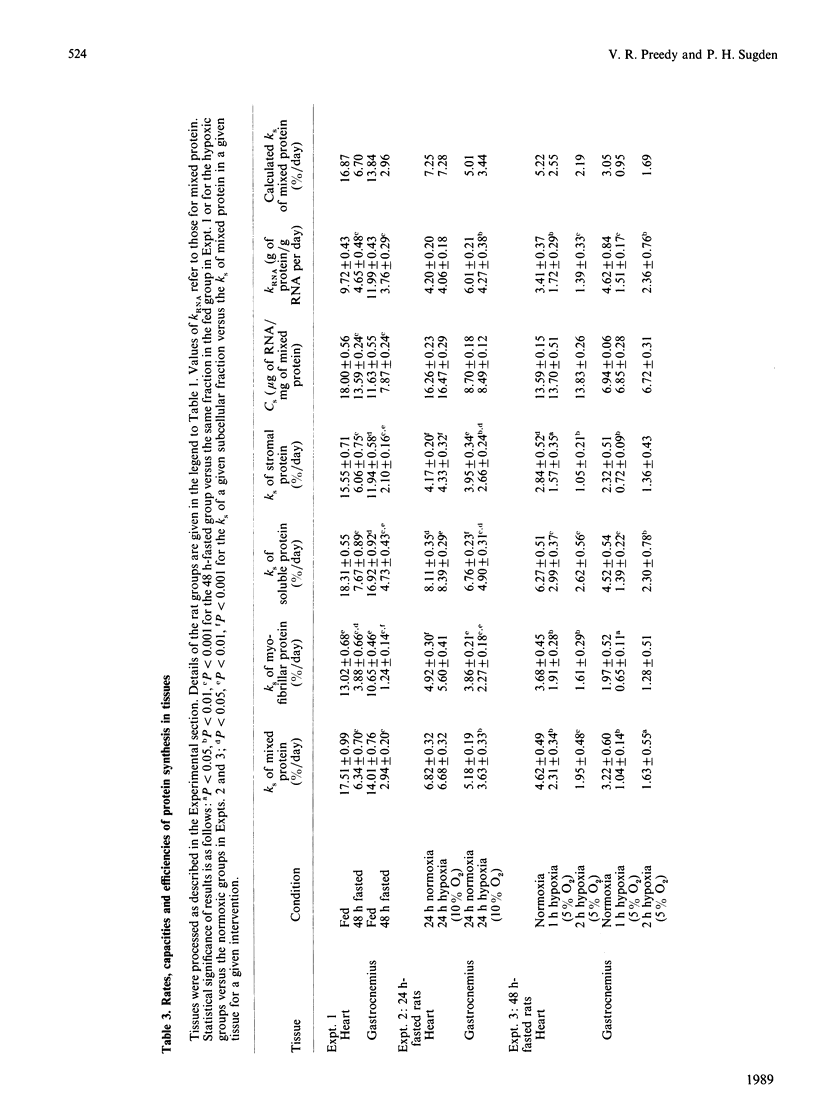
We measured rates of protein synthesis in vivo in subcellular fractions (soluble, myofibrillar and stromal fractions) of the heart and the gastrocnemius from rats after fasting or under hypoxic conditions (i.e. atmospheres containing 5% or 10% O2). Such interventions are known to inhibit protein synthesis under some circumstances. The recovery of tissue protein after fractionation was 80-100%. The proportions of protein present in the soluble and stromal fractions were different in the two muscles. The rates of protein synthesis in the myofibrillar and stromal fractions were less than those for total mixed tissue protein, whereas the rate for soluble protein was greater. Both fasting and moderate hypoxia (10% O2 for 24 h) inhibited protein synthesis in the gastrocnemius. In this tissue, the synthesis of the myofibrillar fraction was apparently the most sensitive to inhibition, and this resulted in some significant increases in the soluble-fraction/myofibrillar-fraction protein-synthesis rate ratios. In the heart, fasting inhibited protein synthesis, but moderate hypoxia (10% O2 for 24 h) did not. The rate of protein synthesis in the cardiac myofibrillar fraction was again more sensitive to fasting than were the rates in the other fractions, but it was not as sensitive as that in the gastrocnemius. Under severely hypoxic conditions (5% O2 for 1 or 2 h), protein synthesis was decreased in all fractions in both tissues. These results suggest that the rates of protein synthesis in these relatively crude subcellular fractions vary.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates P. C., Grimble G. K., Sparrow M. P., Millward D. J. Myofibrillar protein turnover. Synthesis of protein-bound 3-methylhistidine, actin, myosin heavy chain and aldolase in rat skeletal muscle in the fed and starved states. Biochem J. 1983 Aug 15;214(2):593–605. doi: 10.1042/bj2140593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P. C., Millward D. J. Myofibrillar protein turnover. Synthesis rates of myofibrillar and sarcoplasmic protein fractions in different muscles and the changes observed during postnatal development and in response to feeding and starvation. Biochem J. 1983 Aug 15;214(2):587–592. doi: 10.1042/bj2140587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartledge T. G., Lloyd D. Changes in enzyme activities and distributions during glucose de-repression and respiratory adaptation of anaerobically grown Saccharomyces carlsbergensis. Biochem J. 1973 Mar;132(3):609–621. doi: 10.1042/bj1320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Zak R., Fischman D. A., Rabinowitz M. Isolation of newly synthesised myosin filaments from skeletal muscle homogenates and myofibrils. Nature. 1975 May 15;255(5505):259–261. doi: 10.1038/255259a0. [DOI] [PubMed] [Google Scholar]
- FRITTS H. W., Jr, HARRIS P., CLAUSS R. H., ODELL J. E., COURNAND A. The effect of acetylcholine on the human pulmonary circulation under normal and hypoxic conditions. J Clin Invest. 1958 Jan;37(1):99–110. doi: 10.1172/JCI103590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P., Waterlow J. C. The effect of protein deprivation and starvation on the rate of protein synthesis in tissues of the rat. Biochim Biophys Acta. 1975 Nov 18;414(1):71–84. doi: 10.1016/0005-2787(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Genovese A., Chiariello M., De Alfieri W., Latte S., Condorelli M. Production of cardiac lesions with tyramine in intact rats. Studies on serum and myocardial creatine kinase activity changes and ultrastructural aspects. Basic Res Cardiol. 1983 May-Jun;78(3):289–297. doi: 10.1007/BF01907438. [DOI] [PubMed] [Google Scholar]
- Genovese A., De Alfieri W., Latte S., Chiariello M., Condorelli M. Regression of myocardial hypertrophy in the rat following removal of acute or chronic hypobaric hypoxia. Eur Heart J. 1982 Apr;3 (Suppl A):161–164. doi: 10.1093/eurheartj/3.suppl_a.161. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Ruderman N. B. Starvation in the rat. I. Effect of age and obesity on organ weights, RNA, DNA, and protein. Am J Physiol. 1980 Oct;239(4):E269–E276. doi: 10.1152/ajpendo.1980.239.4.E269. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Bates P. C., Millward D. J. Turnover of muscle protein in the fowl (Gallus domesticus). Rates of protein synthesis in fast and slow skeletal, cardiac and smooth muscle of the adult fowl. Biochem J. 1978 Nov 15;176(2):393–401. doi: 10.1042/bj1760393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobley G. E., Lovie J. M. The synthesis of myosin, actin and the major protein fractions in rabbit skeletal muscle. Biochem J. 1979 Sep 15;182(3):867–874. doi: 10.1042/bj1820867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. F. Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem. 1981 Jan 25;256(2):964–968. [PubMed] [Google Scholar]
- Millward D. J., Nnanyelugo D. O., James W. P., Garlick P. J. Protein metabolism in skeletal muscle: the effect of feeding and fasting on muscle RNA, free amino acids and plasma insulin concentrations. Br J Nutr. 1974 Jul;32(1):127–142. doi: 10.1079/bjn19740063. [DOI] [PubMed] [Google Scholar]
- Pomposelli J. J., Palombo J. D., Hamawy K. J., Bistrian B. R., Blackburn G. L., Moldawer L. L. Comparison of different techniques for estimating rates of protein synthesis in vivo in healthy and bacteraemic rats. Biochem J. 1985 Feb 15;226(1):37–42. doi: 10.1042/bj2260037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy V. R., Garlick P. J. Protein synthesis in skeletal muscle of the perfused rat hemicorpus compared with rates in the intact animal. Biochem J. 1983 Aug 15;214(2):433–442. doi: 10.1042/bj2140433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy V. R., Paska L., Sugden P. H., Schofield P. S., Sugden M. C. The effects of surgical stress and short-term fasting on protein synthesis in vivo in diverse tissues of the mature rat. Biochem J. 1988 Feb 15;250(1):179–188. doi: 10.1042/bj2500179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy V. R., Smith D. M., Kearney N. F., Sugden P. H. Rates of protein turnover in vivo and in vitro in ventricular muscle of hearts from fed and starved rats. Biochem J. 1984 Sep 1;222(2):395–400. doi: 10.1042/bj2220395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy V. R., Smith D. M., Sugden P. H. The effects of 6 hours of hypoxia on protein synthesis in rat tissues in vivo and in vitro. Biochem J. 1985 May 15;228(1):179–185. doi: 10.1042/bj2280179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Sugden P. H. Rates of synthesis of actomyosin in atria and ventricles of the perfused working rat heart. Cardiovasc Res. 1985 Sep;19(9):552–558. doi: 10.1093/cvr/19.9.552. [DOI] [PubMed] [Google Scholar]
- Zak R., Martin A. F., Prior G., Rabinowitz M. Comparison of turnover of several myofibrillar proteins and critical evaluation of double isotope method. J Biol Chem. 1977 May 25;252(10):3430–3435. [PubMed] [Google Scholar]


