Abstract
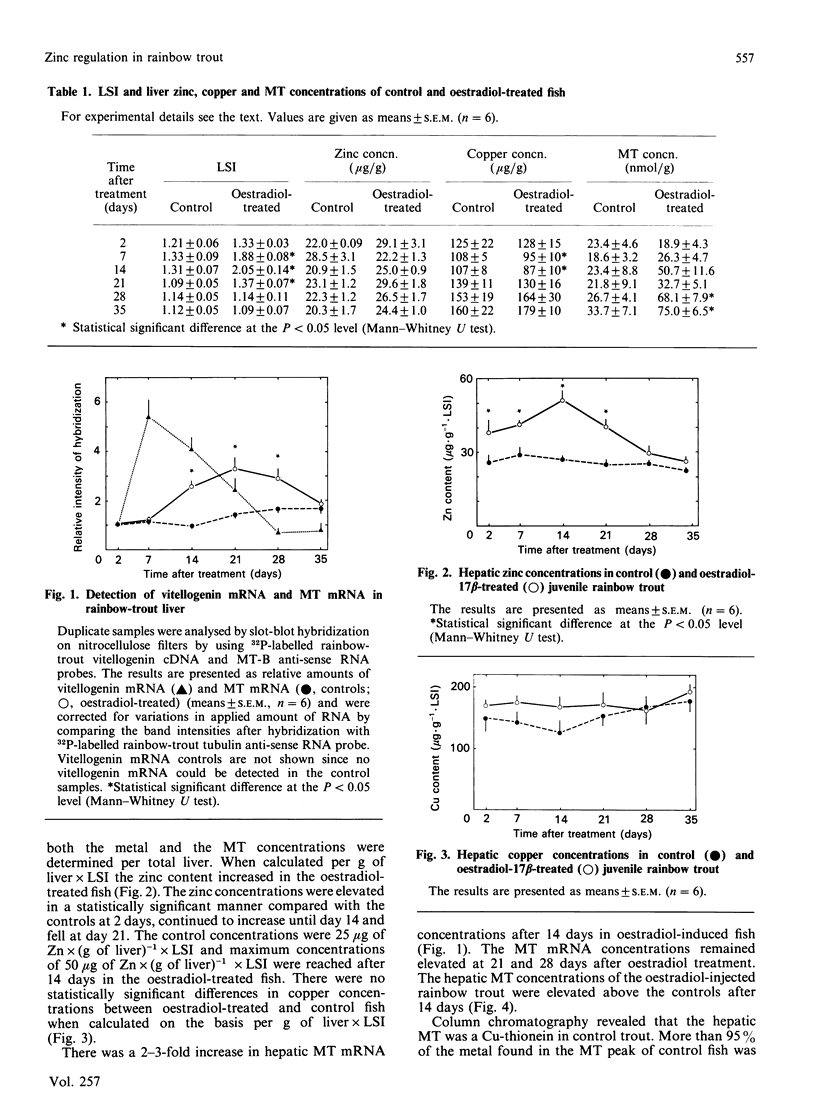
The regulation of metallothionein (MT) biosynthesis in rainbow-trout liver was studied after a single intraperitoneal injection of oestradiol-17 beta. Sampling was performed after 2, 7, 14, 21, 28 and 35 days. Following induction of vitellogenin synthesis in the liver, liver somatic index (LSI) rose from 1.25 to 2.00 in 14 days. Associated with the increase in LSI was an elevation of hepatic vitellogenin mRNA and zinc concentrations. The vitellogenin mRNA concentrations peaked at 7 days after treatment. The zinc concentrations increased to a peak at day 14. MT was analysed by using differential pulse polarography and a rainbow-trout MT RNA probe. The MT mRNA concentrations rose after 14 days and remained elevated at 21 and 28 days. The MT concentrations increased after 14 days and remained elevated throughout the experimental period. The concentrations of MT-bound zinc increased in association with the elevation in MT concentrations in the oestradiol-treated rainbow trout. These findings indicate that MT is involved in the regulation of zinc during the period of vitellogenin induction and that MT may function by maintaining the pool of available zinc at an appropriate concentration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bonham K., Zafarullah M., Gedamu L. The rainbow trout metallothioneins: molecular cloning and characterization of two distinct cDNA sequences. DNA. 1987 Dec;6(6):519–528. doi: 10.1089/dna.1987.6.519. [DOI] [PubMed] [Google Scholar]
- Chvapil M. New aspects in the biological role of zinc: a stabilizer of macromolecules and biological membranes. Life Sci. 1973 Oct 16;13(8):1041–1049. doi: 10.1016/0024-3205(73)90372-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Karin M., Andersen R. D., Slater E., Smith K., Herschman H. R. Metallothionein mRNA induction in HeLa cells in response to zinc or dexamethasone is a primary induction response. Nature. 1980 Jul 17;286(5770):295–297. doi: 10.1038/286295a0. [DOI] [PubMed] [Google Scholar]
- Lei K. Y., Prasad A. S., Bowersox E., Oberleas D. Oral contraceptives, norethindrone and mestranol: effects on tissue levels of minerals. Am J Physiol. 1976 Jul;231(1):98–103. doi: 10.1152/ajplegacy.1976.231.1.98. [DOI] [PubMed] [Google Scholar]
- Oh S. H., Deagen J. T., Whanger P. D., Weswig P. H. Biological function of metallothionein. V. Its induction in rats by various stresses. Am J Physiol. 1978 Mar;234(3):E282–E285. doi: 10.1152/ajpendo.1978.234.3.E282. [DOI] [PubMed] [Google Scholar]
- Olafson R. W. Thymus metallothionein: regulation of zinc-thionein in the aging mouse. Can J Biochem Cell Biol. 1985 Feb;63(2):91–95. doi: 10.1139/o85-013. [DOI] [PubMed] [Google Scholar]
- Panemangalore M., Banerjee D., Onosaka S., Cherian M. G. Changes in the intracellular accumulation and distribution of metallothionein in rat liver and kidney during postnatal development. Dev Biol. 1983 May;97(1):95–102. doi: 10.1016/0012-1606(83)90067-2. [DOI] [PubMed] [Google Scholar]
- Sandstead H. H. Some trace elements which are essential for human nutrition: zinc, copper, manganese, and chromium. Prog Food Nutr Sci. 1975;1(6):371–391. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Varshney U., Gedamu L. Human metallothionein MT-I and MT-II processed genes. Gene. 1984 Nov;31(1-3):135–145. doi: 10.1016/0378-1119(84)90204-x. [DOI] [PubMed] [Google Scholar]
- van Bohemen C. G., Lambert J. G., Peute J. Annual changes in plasma and liver in relation to vitellogenesis in the female rainbow trout, Salmo gairdneri. Gen Comp Endocrinol. 1981 May;44(1):94–107. doi: 10.1016/0016-6480(81)90360-9. [DOI] [PubMed] [Google Scholar]


