Abstract
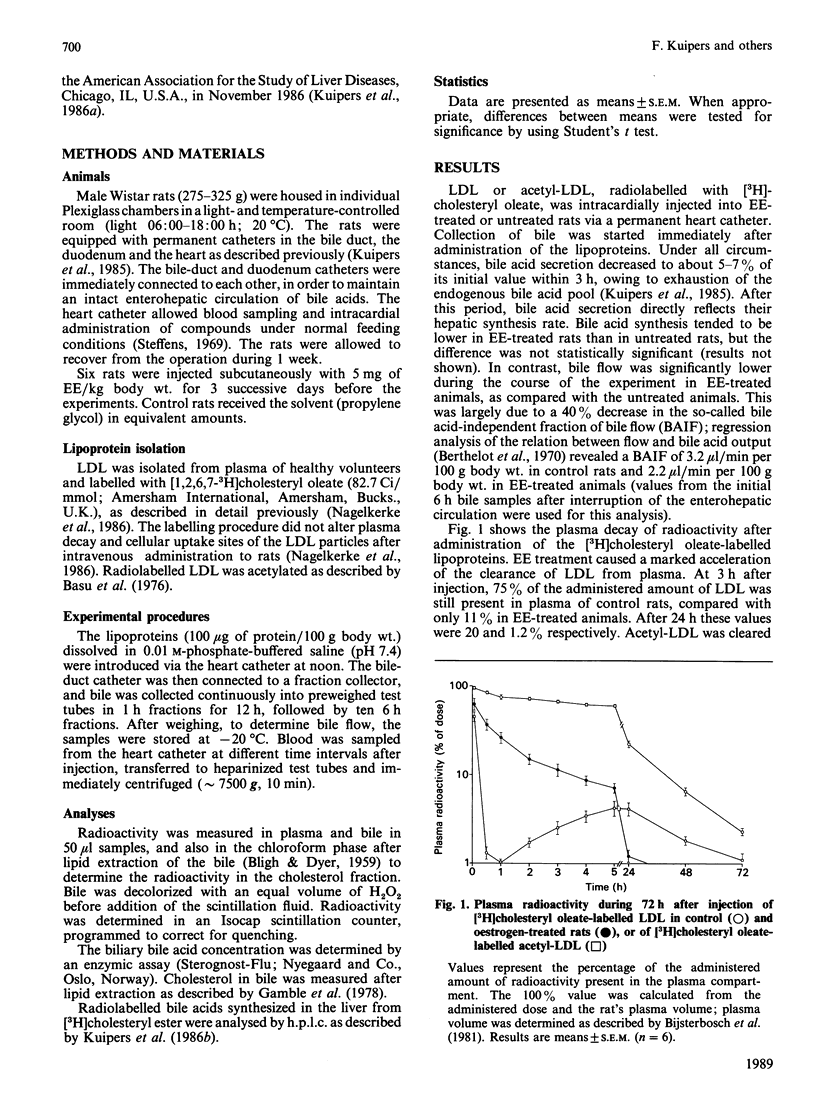
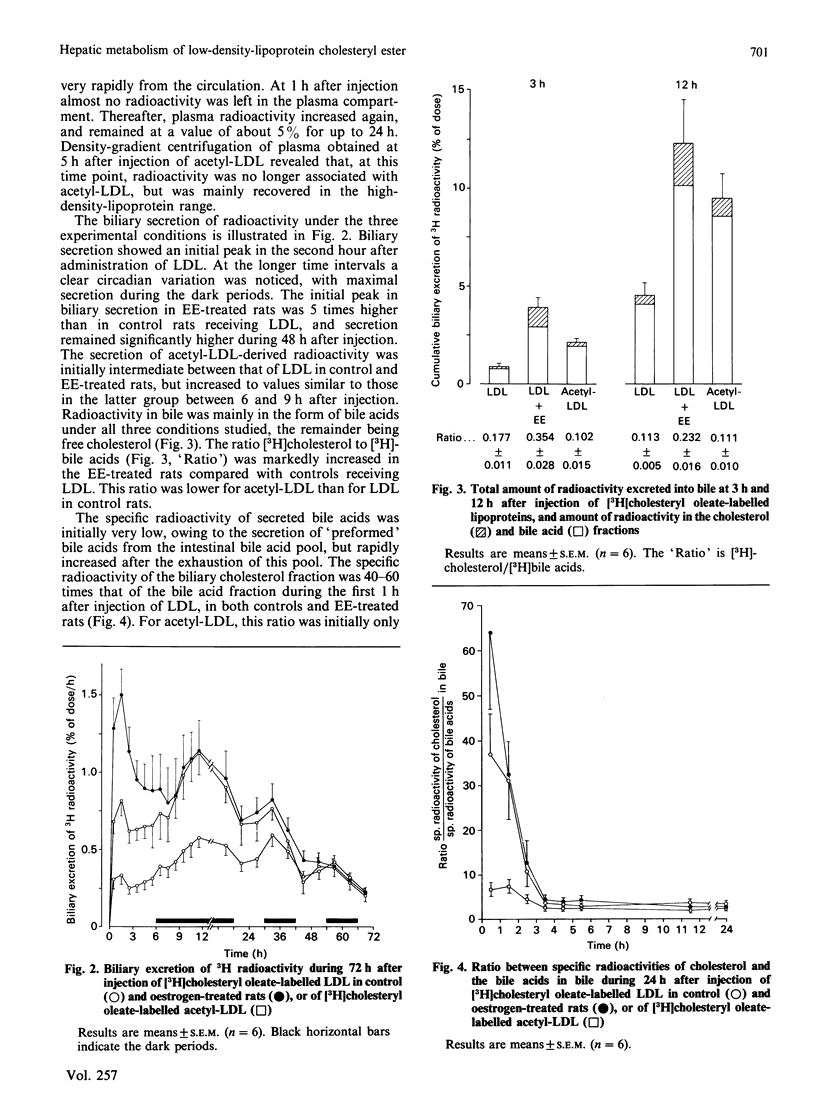
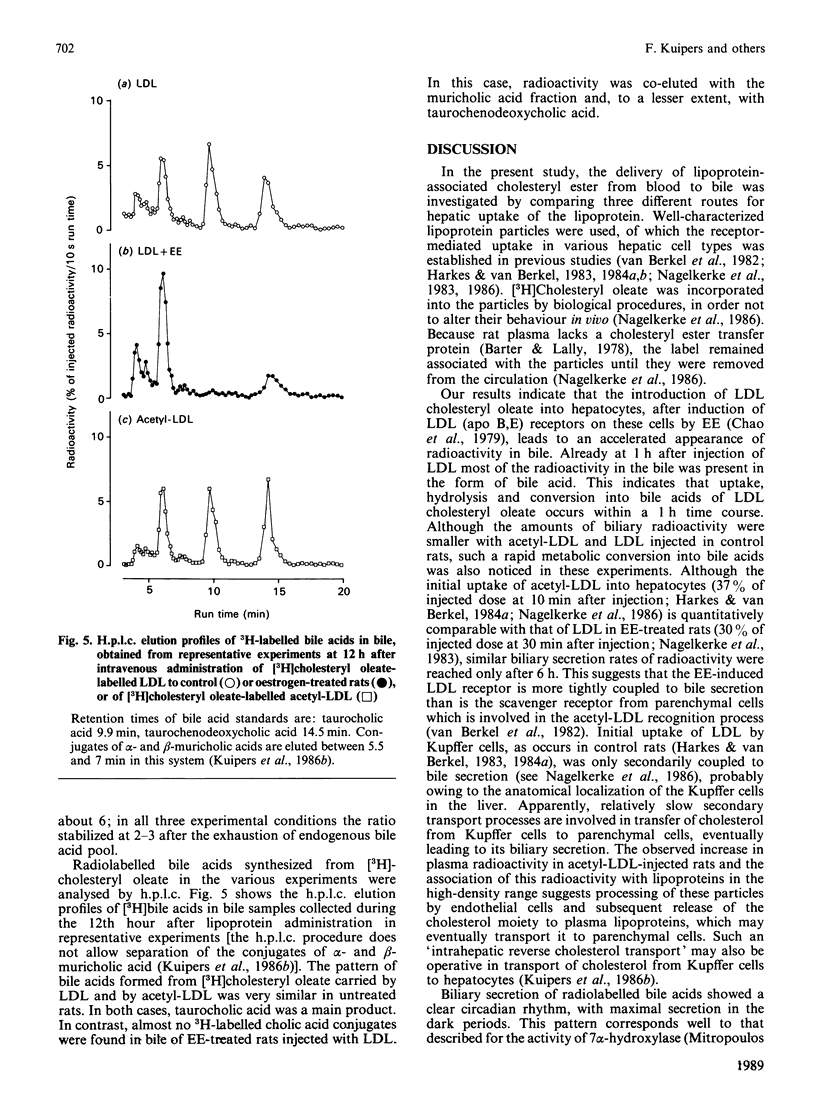
Biliary secretion of the cholesteryl ester moiety of (modified) low-density lipoprotein (LDL) was examined under various experimental conditions in the rat. Human LDL or acetylated LDL (acetyl-LDL), radiolabelled with [3H]cholesteryl oleate, was administered intravenously to unanesthetized rats equipped with permanent catheters in the bile duct, duodenum and heart. LDL was cleared relatively slowly from plasma, mainly by Kupffer cells. At 3 h after injection, only 0.9% of the radioactivity was found in bile; after 12 h this value was 4.5%. Uptake of LDL by hepatocytes was stimulated by treatment of the rats with 17 alpha-ethinyloestradiol (EE; 5 mg/kg for 3 successive days); this resulted in a more rapid secretion of radioactivity into bile, 3.9% and 12.4% after 3 h and 12 h respectively. The extremely rapid uptake of acetyl-LDL via the scavenger pathway, mainly by endothelial cells, resulted in the secretion of only 2.1% of its 3H label into bile within 3 h, and 9.5% within 12 h. Radioactivity in bile was predominantly in the form of bile acids; only a small part was secreted as free cholesterol. However, the specific radioactivity of biliary cholesterol was higher than that of bile acids in all three experimental conditions. EE-treated animals did not form cholic acid from [3H]cholesteryl oleate, which was a major product of the cholesteryl oleate from LDL and acetyl-LDL in untreated rats, but formed predominantly very polar bile acids, i.e. muricholic acids. It is concluded that uptake of human LDL or acetyl-LDL by the liver of untreated rats is not efficiently coupled to biliary secretion of cholesterol (bile acids). This might be due to the anatomical localization of their principal uptake sites, the Kupffer cells and the endothelial cells respectively. Induction of LDL uptake by hepatocytes by EE treatment warrants a more efficient disposition of cholesterol from the body via bile.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attie A. D., Pittman R. C., Steinberg D. Hepatic catabolism of low density lipoprotein: mechanisms and metabolic consequences. Hepatology. 1982 Mar-Apr;2(2):269–281. doi: 10.1002/hep.1840020215. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barter P. J., Lally J. I. The activity of an esterified cholesterol transferring factor in human and rat serum. Biochim Biophys Acta. 1978 Nov 22;531(2):233–236. doi: 10.1016/0005-2760(78)90147-9. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot P., Erlinger S., Dhumeaux D., Preaux A. M. Mechanism of phenobarbital-induced hypercholeresis in the rat. Am J Physiol. 1970 Sep;219(3):809–813. doi: 10.1152/ajplegacy.1970.219.3.809. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Balasubramaniam S., Simons L. A. Quantification of LDL cholesteryl ester contribution to biliary steroids in the rat. Biochim Biophys Acta. 1986 May 21;876(3):413–416. doi: 10.1016/0005-2760(86)90027-5. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Duursma A. M., Bouma J. M., Gruber M. The plasma volume of the Wistar rat in relation to the body weight. Experientia. 1981 Apr 15;37(4):381–382. doi: 10.1007/BF01959874. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Danielsson H., Gustafsson J. On the effect of thyroid hormone on 26-hydroxylation of C 27 -steroids in rat liver. FEBS Lett. 1973 Apr 1;31(1):20–22. doi: 10.1016/0014-5793(73)80064-x. [DOI] [PubMed] [Google Scholar]
- Bonorris G. G., Coyne M. J., Chung A., Schoenfield L. J. Mechanism of estrogen-induced saturated bile in the hamster. J Lab Clin Med. 1977 Dec;90(6):963–970. [PubMed] [Google Scholar]
- Chao Y. S., Windler E. E., Chen G. C., Havel R. J. Hepatic catabolism of rat and human lipoproteins in rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11360–11366. [PubMed] [Google Scholar]
- Davis R. A., Elliott T. S., Lattier G. R., Showalter R. B., Kern F., Jr Regulation of bile acid synthesis via direct effects on the microsomal membrane. Biochemistry. 1986 Apr 8;25(7):1632–1636. doi: 10.1021/bi00355a028. [DOI] [PubMed] [Google Scholar]
- Davis R. A., Showalter R., Kern F., Jr Reversal by triton WR-1339 of ethynyloestradiol-induced hepatic cholesterol esterification. Biochem J. 1978 Jul 15;174(1):45–51. doi: 10.1042/bj1740045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M. Regulation of cholesterol metabolism in man and in other species. Klin Wochenschr. 1984 Apr 16;62(8):338–345. doi: 10.1007/BF01716251. [DOI] [PubMed] [Google Scholar]
- Gumucio J. J., Valdivieso V. D. Studies on the mechanism of the ethynylestradiol impairment of bile flow and bile salt excretion in the rat. Gastroenterology. 1971 Sep;61(3):339–344. [PubMed] [Google Scholar]
- Harkes L., Van Berkel J. C. Quantitative role of parenchymal and non-parenchymal liver cells in the uptake of [14C]sucrose-labelled low-density lipoprotein in vivo. Biochem J. 1984 Nov 15;224(1):21–27. doi: 10.1042/bj2240021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkes L., Van Berkel T. J. In vivo characteristics of a specific recognition site for LDL on non-parenchymal rat liver cells which differs from the 17 alpha-ethinyl estradiol-induced LDL receptor on parenchymal liver cells. Biochim Biophys Acta. 1984 Jul 6;794(2):340–347. doi: 10.1016/0005-2760(84)90165-6. [DOI] [PubMed] [Google Scholar]
- Harkes L., van Berkel T. J. Cellular localization of the receptor-dependent and receptor-independent uptake of human LDL in the liver of normal and 17 alpha-ethinyl estradiol-treated rats. FEBS Lett. 1983 Apr 5;154(1):75–80. doi: 10.1016/0014-5793(83)80878-3. [DOI] [PubMed] [Google Scholar]
- Ho K. J., Drummond J. L. Circadian rhythm of biliary excretion and its control mechanisms in rats with chronic biliary drainage. Am J Physiol. 1975 Nov;229(5):1427–1437. doi: 10.1152/ajplegacy.1975.229.5.1427. [DOI] [PubMed] [Google Scholar]
- Kern F., Jr, Eriksson H., Curstedt T., Sjövall J. Effect of ethynylestradiol on biliary excretion of bile acids, phosphatidylcolines, and cholesterol in the bile fistula rat. J Lipid Res. 1977 Sep;18(5):623–634. [PubMed] [Google Scholar]
- Kuipers F., Havinga R., Bosschieter H., Toorop G. P., Hindriks F. R., Vonk R. J. Enterohepatic circulation in the rat. Gastroenterology. 1985 Feb;88(2):403–411. doi: 10.1016/0016-5085(85)90499-8. [DOI] [PubMed] [Google Scholar]
- Kuipers F., Spanjer H. H., Havinga R., Scherphof G. L., Vonk R. J. Lipoproteins and liposomes as in vivo cholesterol vehicles in the rat: preferential use of cholesterol carried by small unilamellar liposomes for the formation of muricholic acids. Biochim Biophys Acta. 1986 May 21;876(3):559–566. doi: 10.1016/0005-2760(86)90044-5. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Balasubramaniam S., Myant N. B. The effect of interruption of the enterophecatic circulation of bile acids and of cholesterol feeding on cholesterol 7 alpha-hydroxylase in relation to the diurnal rhythm in its activity. Biochim Biophys Acta. 1973 Dec 20;326(3):428–438. doi: 10.1016/0005-2760(73)90143-4. [DOI] [PubMed] [Google Scholar]
- Nagelkerke J. F., Bakkeren H. F., Kuipers F., Vonk R. J., van Berkel T. J. Hepatic processing of the cholesteryl ester from low density lipoprotein in the rat. J Biol Chem. 1986 Jul 5;261(19):8908–8913. [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Nagelkerke J. F., van Berkel T. J. Rapid transport of fatty acids from rat liver endothelial to parenchymal cells after uptake of cholesteryl ester-labeled acetylated LDL. Biochim Biophys Acta. 1986 Feb 28;875(3):593–598. doi: 10.1016/0005-2760(86)90081-0. [DOI] [PubMed] [Google Scholar]
- Packard C. J., Shepherd J. The hepatobiliary axis and lipoprotein metabolism: effects of bile acid sequestrants and ileal bypass surgery. J Lipid Res. 1982 Nov;23(8):1081–1098. [PubMed] [Google Scholar]
- Spady D. K., Turley S. D., Dietschy J. M. Receptor-independent low density lipoprotein transport in the rat in vivo. Quantitation, characterization, and metabolic consequences. J Clin Invest. 1985 Sep;76(3):1113–1122. doi: 10.1172/JCI112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berkel T. J., Nagelkerke J. F., Harkes L., Kruijt J. K. Processing of acetylated human low-density lipoprotein by parenchymal and non-parenchymal liver cells. Involvement of calmodulin? Biochem J. 1982 Nov 15;208(2):493–503. doi: 10.1042/bj2080493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk R. J., van Doorn A. B., Strubbe J. H. Bile secretion and bile composition in the freely moving, unanaesthetized rat with a permanent biliary drainage: influence of food intake on bile flow. Clin Sci Mol Med. 1978 Sep;55(3):253–259. doi: 10.1042/cs0550253. [DOI] [PubMed] [Google Scholar]


