Abstract
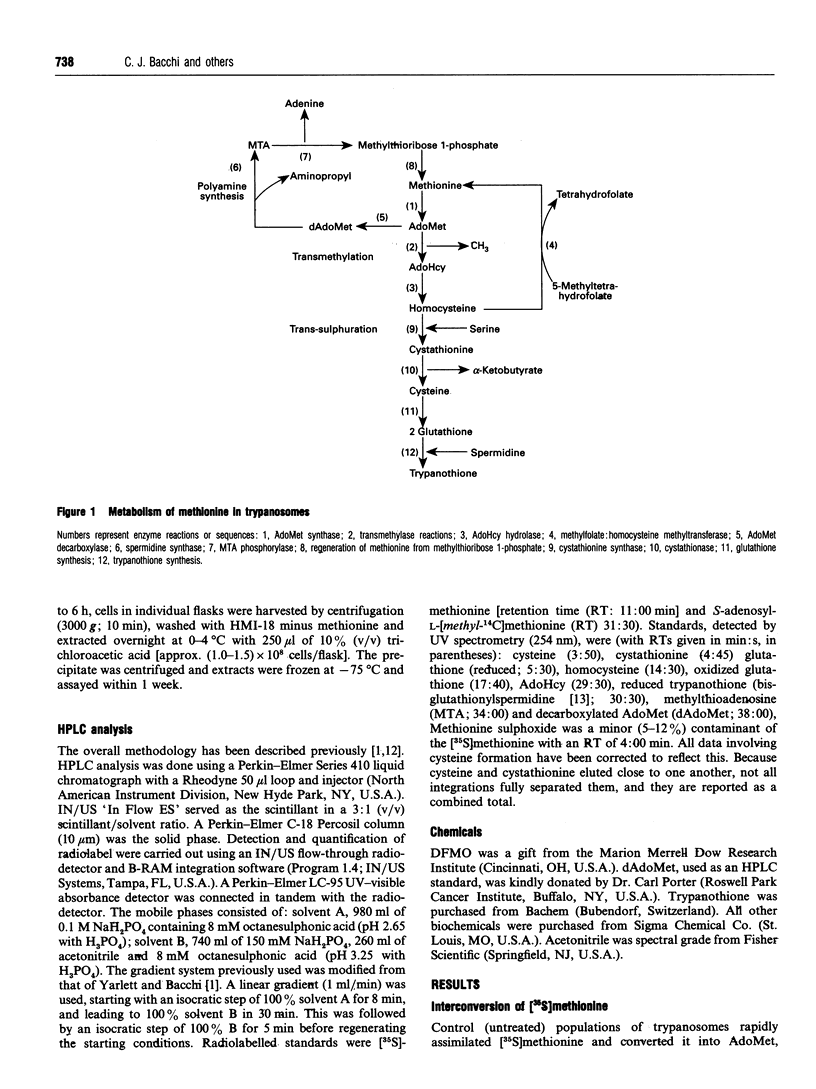
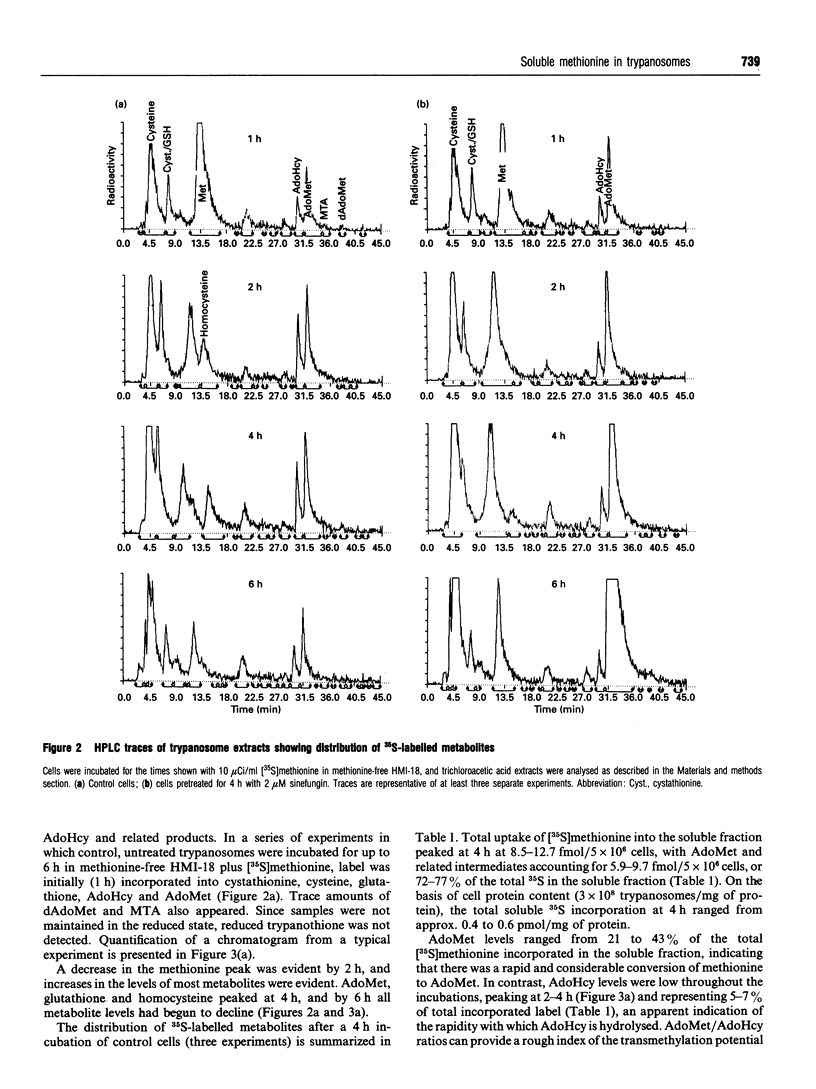
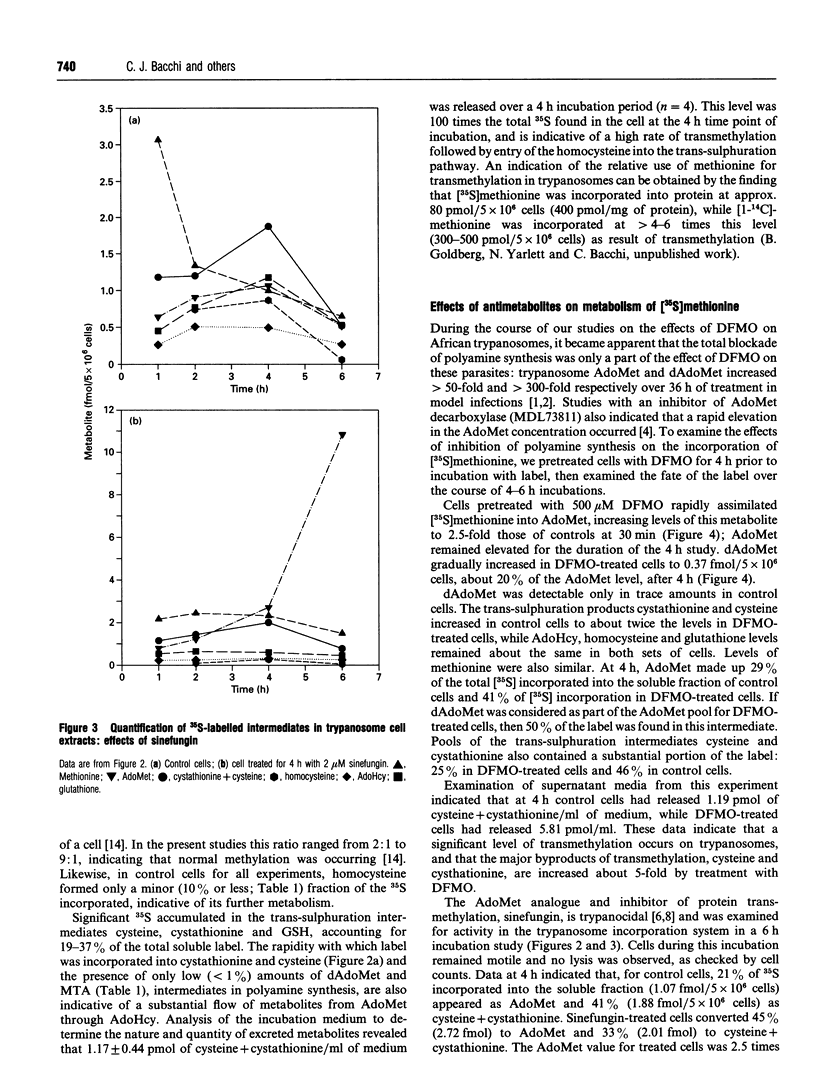
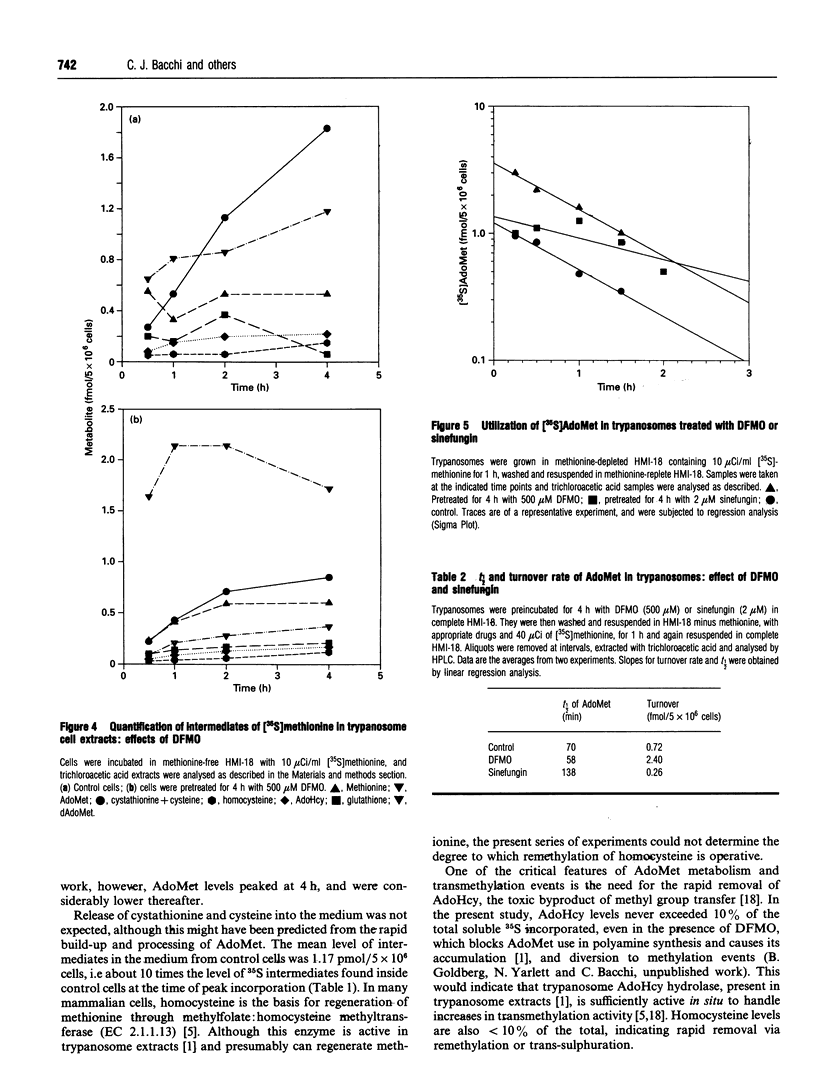
The metabolism of [35S]methionine in cultured bloodstream forms of African trypanosomes was followed using flow-through radiodetection linked to liquid chromatography separation. The effects of a transmethylase inhibitor, sinefungin, and of the ornithine decarboxylase inhibitor, DL-alpha-difluoromethylornithine (Ornidyl; DFMO), on methionine metabolism were also observed. Trypanosomes rapidly incorporated [35S]methionine into S-adenosylmethionine (AdoMet) and the metabolites methylthioadenosine, S-adenosylhomocysteine, homocysteine, cystathionine cysteine and glutathione. Untreated trypanosomes excreted large quantities of cystathionine and cysteine into the growth medium. DFMO-treated cells formed larger quantities of AdoMet more rapidly than did control cells, as was evident from initial time points (30 min and 1 h). Decarboxylated AdoMet, present in trace quantities in control cells, accumulated in DFMO-treated cells. Sinefungin increased the AdoMet concentrations approximately 20-fold over that of controls after a 6 h incubation with [35S]methionine, while cystathionine and cysteine levels decreased. The half-life (t1/2) and rate of turnover of AdoMet were measured in cells treated with DFMO or sinefungin. DFMO treatment caused a substantial increase in the rate of AdoMet utilization, while sinefungin extended the t1/2 and lowered AdoMet turnover. These studies show that trypanosomes rapidly metabolize methionine through AdoMet to intermediates of the polyamine and transmethylation pathways. Agents inhibiting these pathways rapidly affect the concentration and rate of utilization of AdoMet, significantly changing the concentrations of metabolites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila J. L., Polegre M. A. Uptake and metabolism of S-adenosyl-L-methionine by Leishmania mexicana and Leishmania braziliensis promastigotes. Mol Biochem Parasitol. 1993 Mar;58(1):123–134. doi: 10.1016/0166-6851(93)90096-g. [DOI] [PubMed] [Google Scholar]
- Bacchi C. J., Berens R. L., Nathan H. C., Klein R. S., Elegbe I. A., Rao K. V., McCann P. P., Marr J. J. Synergism between 9-deazainosine and DL-alpha-difluoromethylornithine in treatment of experimental African trypanosomiasis. Antimicrob Agents Chemother. 1987 Sep;31(9):1406–1413. doi: 10.1128/aac.31.9.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi C. J., Nathan H. C., Hutner S. H., McCann P. P., Sjoerdsma A. Polyamine metabolism: a potential therapeutic target in trypanosomes. Science. 1980 Oct 17;210(4467):332–334. doi: 10.1126/science.6775372. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Byers T. L., Bush T. L., Casara P. J., Bacchi C. J., Clarkson A. B., Jr, McCann P. P., Sjoerdsma A. Cure of Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense infections in mice with an irreversible inhibitor of S-adenosylmethionine decarboxylase. Antimicrob Agents Chemother. 1990 Aug;34(8):1485–1490. doi: 10.1128/aac.34.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. L., Bush T. L., McCann P. P., Bitonti A. J. Antitrypanosomal effects of polyamine biosynthesis inhibitors correlate with increases in Trypanosoma brucei brucei S-adenosyl-L-methionine. Biochem J. 1991 Mar 1;274(Pt 2):527–533. doi: 10.1042/bj2740527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube D. K., Mpimbaza G., Allison A. C., Lederer E., Rovis L. Antitrypanosomal activity of sinefungin. Am J Trop Med Hyg. 1983 Jan;32(1):31–33. doi: 10.4269/ajtmh.1983.32.31. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- Finkelstein J. D. Methionine metabolism in mammals. J Nutr Biochem. 1990 May;1(5):228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- German D. C., Bloch C. A., Kredich N. M. Measurements of S-adenosylmethionine and L-homocysteine metabolism in cultured human lymphoid cells. J Biol Chem. 1983 Sep 25;258(18):10997–11003. [PubMed] [Google Scholar]
- Hirumi H., Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989 Dec;75(6):985–989. [PubMed] [Google Scholar]
- Hoffman R. M. Altered methionine metabolism and transmethylation in cancer. Anticancer Res. 1985 Jan-Feb;5(1):1–30. [PubMed] [Google Scholar]
- Lawrence F., Robert-Gero M. Distribution of macromolecular methylations in promastigotes of Leishmania donovani and impact of sinefungin. J Eukaryot Microbiol. 1993 Sep-Oct;40(5):581–589. doi: 10.1111/j.1550-7408.1993.tb06111.x. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Danzin C., Wagner J., Siat M., Joder-Ohlenbusch A. M., Claverie N. Accumulation of decarboxylated S-adenosyl-L-methionine in mammalian cells as a consequence of the inhibition of putrescine biosynthesis. Eur J Biochem. 1982 Apr;123(3):499–504. doi: 10.1111/j.1432-1033.1982.tb06559.x. [DOI] [PubMed] [Google Scholar]
- Phelouzat M. A., Lawrence F., Moulay L., Borot C., Schaeverbeke J., Schaeverbeke M., Robert-Gero M. Leishmania donovani: antagonistic effect of S-adenosyl methionine on ultrastructural changes and growth inhibition induced by sinefungin. Exp Parasitol. 1992 Mar;74(2):177–187. doi: 10.1016/0014-4894(92)90045-c. [DOI] [PubMed] [Google Scholar]
- Regina M., Korhonen V. P., Smith T. K., Alakuijala L., Eloranta T. O. Methionine toxicity in the rat in relation to hepatic accumulation of S-adenosylmethionine: prevention by dietary stimulation of the hepatic transsulfuration pathway. Arch Biochem Biophys. 1993 Feb 1;300(2):598–607. doi: 10.1006/abbi.1993.1083. [DOI] [PubMed] [Google Scholar]
- Ueland P. M. Pharmacological and biochemical aspects of S-adenosylhomocysteine and S-adenosylhomocysteine hydrolase. Pharmacol Rev. 1982 Sep;34(3):223–253. [PubMed] [Google Scholar]
- Van Nieuwenhove S. Advances in sleeping sickness therapy. Ann Soc Belg Med Trop. 1992;72 (Suppl 1):39–51. [PubMed] [Google Scholar]
- Yarlett N., Bacchi C. J. Effect of DL-alpha-difluoromethylornithine on methionine cycle intermediates in Trypanosoma brucei brucei. Mol Biochem Parasitol. 1988 Jan 1;27(1):1–10. doi: 10.1016/0166-6851(88)90019-9. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Lindmark D. G., Goldberg B., Moharrami M. A., Bacchi C. J. Subcellular localization of the enzymes of the arginine dihydrolase pathway in Trichomonas vaginalis and Tritrichomonas foetus. J Eukaryot Microbiol. 1994 Nov-Dec;41(6):554–559. doi: 10.1111/j.1550-7408.1994.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Quamina A., Bacchi C. J. Protein methylases in Trypanosoma brucei brucei: activities and response to DL-alpha-difluoromethylornithine. J Gen Microbiol. 1991 Mar;137(3):717–724. doi: 10.1099/00221287-137-3-717. [DOI] [PubMed] [Google Scholar]


