Abstract
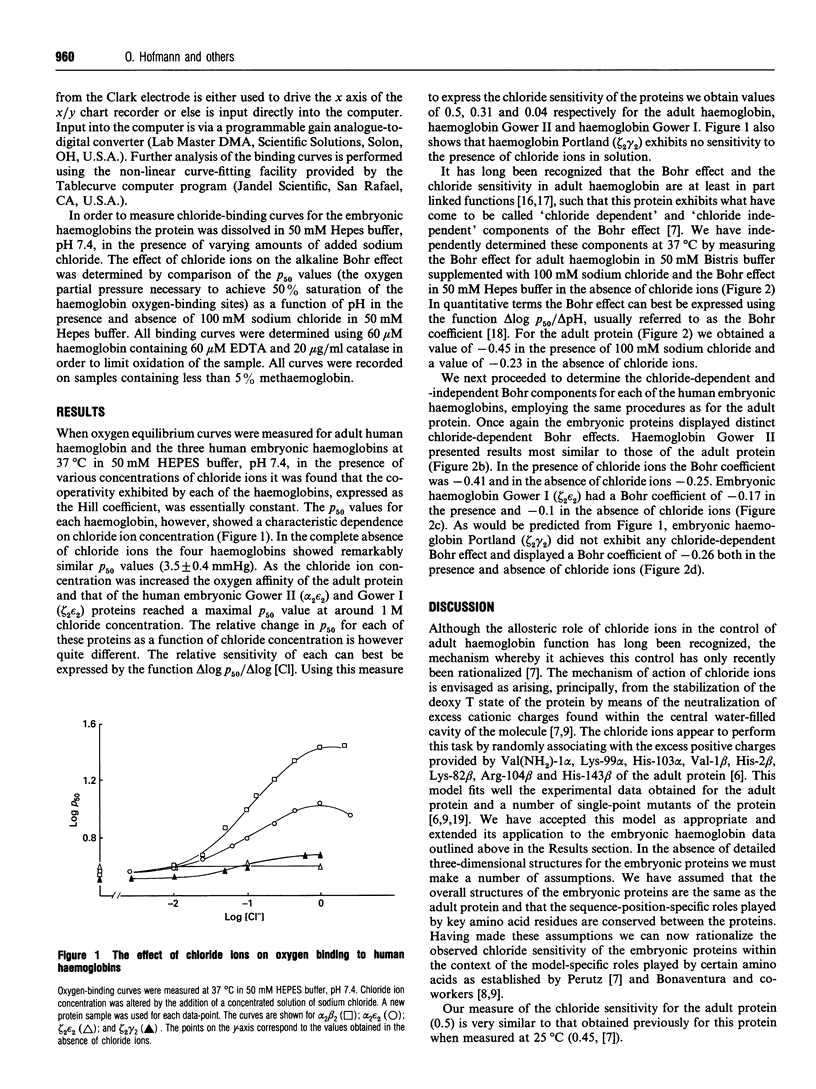
The interactions of the three human embryonic haemoglobins with chloride ions have been investigated. Each of the three embryonic haemoglobins exhibits a unique pattern of oxygen-affinity-dependence on chloride ion concentration. Human embryonic haemoglobin Portland (zeta 2 gamma 2) is found to be completely insensitive to chloride ion concentration. Haemoglobin Gower I (zeta 2 gamma 2) shows a small concentration dependence, whilst haemoglobin Gower II (alpha 2 epsilon 2) exhibits a dependence approaching that of the adult protein. The degree of co-operativity for each protein is essentially chloride concentration independent. The chloride-dependent and -independent components of the alkaline Bohr effects have been measured for each of the embryonic haemoglobins and compared with that of the adult protein. Both the chloride-binding data and the Bohr effect have been analysed in terms of the recently developed allosteric model proposed by Perutz [Perutz, Fermi, Poyart, Pagnier and Kister (1993) J. Mol. Biol. 233, 536-545].
Full text
PDF
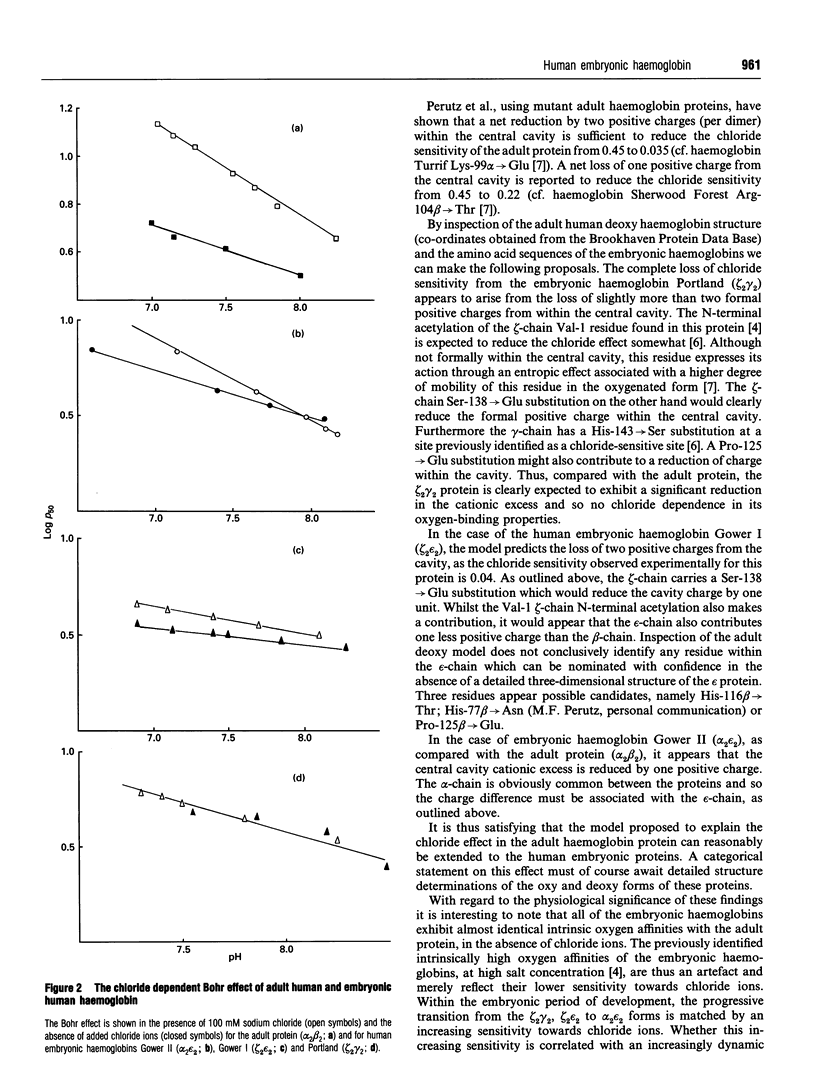

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., WYMAN J., BRUNORI M., BUCCI E., FRONTICELLI C., ROSSI-FANELLI A. STUDIES ON THE RELATIONS BETWEEN MOLECULAR AND FUNCTIONAL PROPERTIES OF HEMOGLOBIN. IV. THE BOHR EFFECT IN HUMAN HEMOGLOBIN MEASURED BY PROTON BINDING. J Biol Chem. 1963 Sep;238:2950–2957. [PubMed] [Google Scholar]
- Bonaventura C., Arumugam M., Cashon R., Bonaventura J., Moo-Penn W. F. Chloride masks effects of opposing positive charges in Hb A and Hb Hinsdale (beta 139 Asn-->Lys) that can modulate cooperativity as well as oxygen affinity. J Mol Biol. 1994 Jun 17;239(4):561–568. doi: 10.1006/jmbi.1994.1395. [DOI] [PubMed] [Google Scholar]
- Bonaventura C., Bonaventura J., Amiconi G., Tentori L., Brunori M., Antonini E. Hemoglobin Abruzzo (beta143 (H21) His replaced by Arg). Consequences of altering the 2,3-diphosphoglycerate binding site. J Biol Chem. 1975 Aug 25;250(16):6273–6277. [PubMed] [Google Scholar]
- Bonaventura J., Bonaventura C., Sullivan B., Ferruzzi G., McCurdy P. R., Fox J., Moo-Penn W. F. Hemoglobin providence. Functional consequences of two alterations of the 2,3-diphosphoglycerate binding site at position beta 82. J Biol Chem. 1976 Dec 10;251(23):7563–7571. [PubMed] [Google Scholar]
- Bonaventura J., Bonaventura C., Sullivan B., Godette G. Hemoglobin Deer Lodge (beta 2 His replaced by Arg). Consequences of altering the 2,3-diphosphoglycerate binding site. J Biol Chem. 1975 Dec 25;250(24):9250–9255. [PubMed] [Google Scholar]
- Hofmann O., Mould R., Brittain T. Allosteric modulation of oxygen binding to the three human embryonic haemoglobins. Biochem J. 1995 Mar 1;306(Pt 2):367–370. doi: 10.1042/bj3060367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehns E. R., Faroqui A. M. Oxygen dissociation properties of human embryonic red cells. Nature. 1975 Mar 27;254(5498):335–337. doi: 10.1038/254335a0. [DOI] [PubMed] [Google Scholar]
- Kavanaugh J. S., Rogers P. H., Case D. A., Arnone A. High-resolution X-ray study of deoxyhemoglobin Rothschild 37 beta Trp----Arg: a mutation that creates an intersubunit chloride-binding site. Biochemistry. 1992 Apr 28;31(16):4111–4121. doi: 10.1021/bi00131a030. [DOI] [PubMed] [Google Scholar]
- Mould R. M., Hofmann O. M., Brittain T. Production of human embryonic haemoglobin (Gower II) in a yeast expression system. Biochem J. 1994 Mar 15;298(Pt 3):619–622. doi: 10.1042/bj2980619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell S., Mandaro R., Schuster T. M., Arnone A. X-ray diffraction and solution studies of specifically carbamylated human hemoglobin A. Evidence for the location of a proton- and oxygen-linked chloride binding site at valine 1 alpha. J Biol Chem. 1979 Dec 10;254(23):12204–12208. [PubMed] [Google Scholar]
- Perutz M. F., Fermi G., Poyart C., Pagnier J., Kister J. A novel allosteric mechanism in haemoglobin. Structure of bovine deoxyhaemoglobin, absence of specific chloride-binding sites and origin of the chloride-linked Bohr effect in bovine and human haemoglobin. J Mol Biol. 1993 Oct 5;233(3):536–545. doi: 10.1006/jmbi.1993.1530. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Shih D. T., Williamson D. The chloride effect in human haemoglobin. A new kind of allosteric mechanism. J Mol Biol. 1994 Jun 17;239(4):555–560. doi: 10.1006/jmbi.1994.1394. [DOI] [PubMed] [Google Scholar]
- Rollema H. S., de Bruin S. H., Janssen L. H., van Os G. A. The effect of potassium chloride on the Bohr effect of human hemoglobin. J Biol Chem. 1975 Feb 25;250(4):1333–1339. [PubMed] [Google Scholar]
- Shaanan B. Structure of human oxyhaemoglobin at 2.1 A resolution. J Mol Biol. 1983 Nov 25;171(1):31–59. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- Shih D. T., Luisi B. F., Miyazaki G., Perutz M. F., Nagai K. A mutagenic study of the allosteric linkage of His(HC3)146 beta in haemoglobin. J Mol Biol. 1993 Apr 20;230(4):1291–1296. doi: 10.1006/jmbi.1993.1242. [DOI] [PubMed] [Google Scholar]


