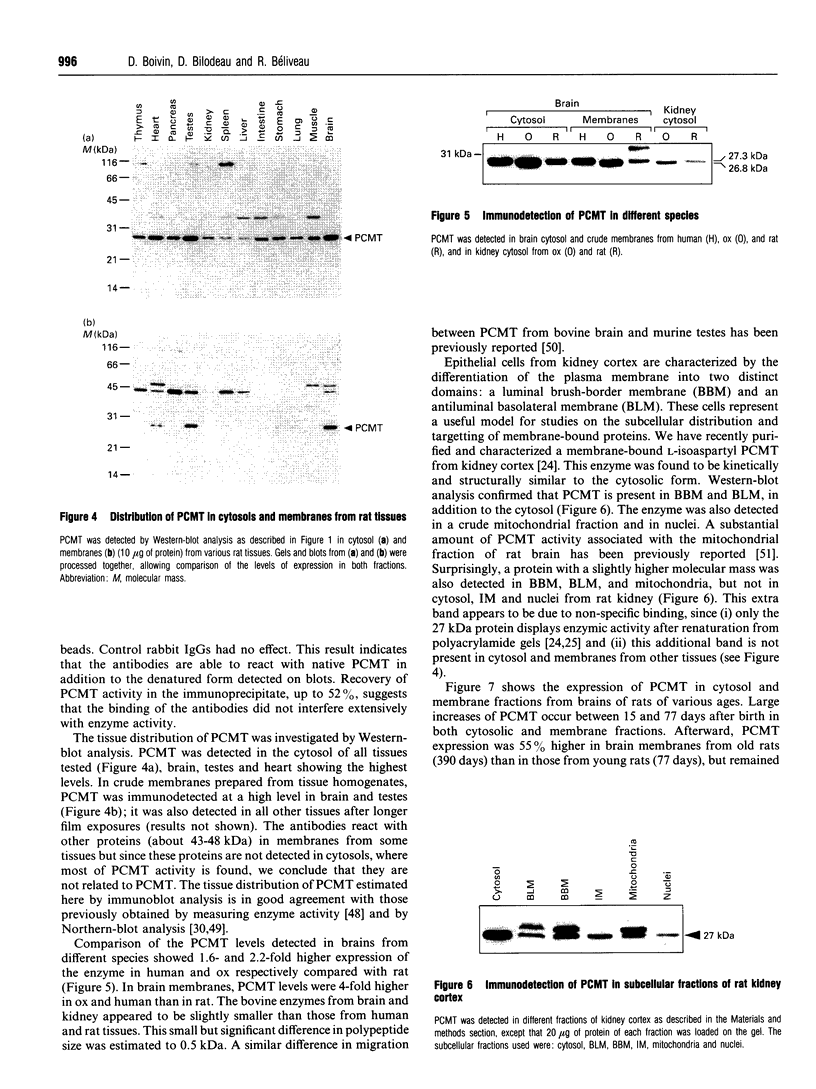
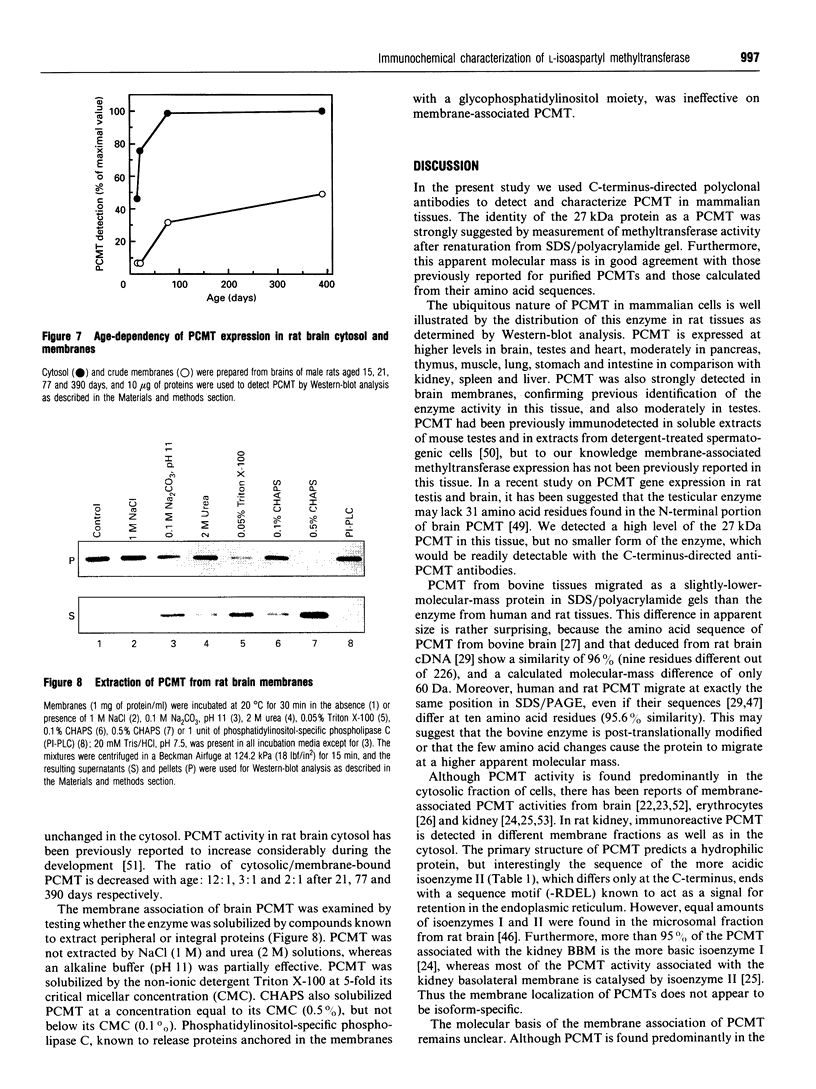
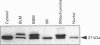Abstract
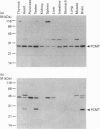
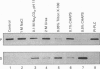
Polyclonal antibodies were raised against a synthetic peptide corresponding to a sequence of 14 amino acid residues found near the C-terminus of L-isoaspartyl (D-aspartyl)-protein carboxyl methyltransferase (PCMT). The affinity-purified antibodies were used to detect the methyltransferase by Western-blot analysis in cytosolic and membrane fractions from several mammalian tissues. A protein of 27 kDa was detected in the cytosol of most tissues; co-incubation with the peptide used for immunization abolished the detection. The identity of the 27 kDa protein as a PCMT was demonstrated by renaturation of PCMT activity from SDS/polyacrylamide gels. The methyltransferase from brain cytosol was immunoprecipitated by the anti-PCMT antibodies and Protein A-agarose, indicating that the native protein was recognized by the antibodies. PCMT was also immunodetected in crude membranes from brain, testes and heart, and in purified membranes from kidney cortex. The expression of the methyltransferase was higher in bovine and human brain than in rat tissues. The bovine enzyme had a greater electrophoretic mobility, suggesting small structural differences. The membrane-bound methyltransferase could be extracted with detergents above their critical micellar concentration, but not with salt, alkaline or urea solutions suggesting that the binding of the enzyme to membranes is hydrophobic by nature. Anti-PCMT antibodies provide an interesting tool for studies regarding the expression of these enzymes in both soluble and membrane fractions of various cell types.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswad D. W., Deight E. A. Purification and characterization of two distinct isozymes of protein carboxymethylase from bovine brain. J Neurochem. 1983 Jun;40(6):1718–1726. doi: 10.1111/j.1471-4159.1983.tb08147.x. [DOI] [PubMed] [Google Scholar]
- Aswad D. W. Stoichiometric methylation of porcine adrenocorticotropin by protein carboxyl methyltransferase requires deamidation of asparagine 25. Evidence for methylation at the alpha-carboxyl group of atypical L-isoaspartyl residues. J Biol Chem. 1984 Sep 10;259(17):10714–10721. [PubMed] [Google Scholar]
- Barten D. M., O'Dea R. F. The function of protein carboxylmethyltransferase in eucaryotic cells. Life Sci. 1990;47(3):181–194. doi: 10.1016/0024-3205(90)90319-m. [DOI] [PubMed] [Google Scholar]
- Boivin D., Gingras D., Béliveau R. Purification and characterization of a membrane-bound protein carboxyl methyltransferase from rat kidney cortex. J Biol Chem. 1993 Feb 5;268(4):2610–2615. [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clarke S. Protein carboxyl methyltransferases: two distinct classes of enzymes. Annu Rev Biochem. 1985;54:479–506. doi: 10.1146/annurev.bi.54.070185.002403. [DOI] [PubMed] [Google Scholar]
- Fu J. C., Ding L., Clarke S. Purification, gene cloning, and sequence analysis of an L-isoaspartyl protein carboxyl methyltransferase from Escherichia coli. J Biol Chem. 1991 Aug 5;266(22):14562–14572. [PubMed] [Google Scholar]
- Geiger T., Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem. 1987 Jan 15;262(2):785–794. [PubMed] [Google Scholar]
- Gilbert J. M., Fowler A., Bleibaum J., Clarke S. Purification of homologous protein carboxyl methyltransferase isozymes from human and bovine erythrocytes. Biochemistry. 1988 Jul 12;27(14):5227–5233. doi: 10.1021/bi00414a042. [DOI] [PubMed] [Google Scholar]
- Gingras D., Boivin D., Beliveau R. Asymmetrical distribution of L-isoaspartyl protein carboxyl methyltransferases in the plasma membranes of rat kidney cortex. Biochem J. 1994 Jan 1;297(Pt 1):145–150. doi: 10.1042/bj2970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras D., Boivin D., Béliveau R. Subcellular distribution and guanine nucleotide dependency of COOH-terminal methylation in kidney cortex. Am J Physiol. 1993 Aug;265(2 Pt 2):F316–F322. doi: 10.1152/ajprenal.1993.265.2.F316. [DOI] [PubMed] [Google Scholar]
- Gingras D., Ménard P., Béliveau R. Protein carboxyl methylation in kidney brush-border membranes. Biochim Biophys Acta. 1991 Jul 22;1066(2):261–267. doi: 10.1016/0005-2736(91)90196-f. [DOI] [PubMed] [Google Scholar]
- Gmaj P., Murer H., Carafoli E. Localization and properties of a high-affinity (Ca2+ + Mg2+)-ATPase in isolated kidney cortex plasma membranes. FEBS Lett. 1982 Aug 2;144(2):226–230. doi: 10.1016/0014-5793(82)80643-1. [DOI] [PubMed] [Google Scholar]
- Hrycyna C. A., Clarke S. Modification of eukaryotic signaling proteins by C-terminal methylation reactions. Pharmacol Ther. 1993 Sep;59(3):281–300. doi: 10.1016/0163-7258(93)90071-k. [DOI] [PubMed] [Google Scholar]
- Ingrosso D., Fowler A. V., Bleibaum J., Clarke S. Sequence of the D-aspartyl/L-isoaspartyl protein methyltransferase from human erythrocytes. Common sequence motifs for protein, DNA, RNA, and small molecule S-adenosylmethionine-dependent methyltransferases. J Biol Chem. 1989 Nov 25;264(33):20131–20139. [PubMed] [Google Scholar]
- Ingrosso D., Kagan R. M., Clarke S. Distinct C-terminal sequences of isozymes I and II of the human erythrocyte L-isoaspartyl/D-aspartyl protein methyltransferase. Biochem Biophys Res Commun. 1991 Feb 28;175(1):351–358. doi: 10.1016/s0006-291x(05)81242-2. [DOI] [PubMed] [Google Scholar]
- Iqbal M., Steenson T. Purification of protein carboxymethylase from ox brain. J Neurochem. 1976 Aug;27(2):605–608. doi: 10.1111/j.1471-4159.1976.tb12289.x. [DOI] [PubMed] [Google Scholar]
- Kelly M. H., Hamilton J. R. A micro-technique for the assay of intestinal alkaline phosphatase. Results in normal children and in children with celiac disease. Clin Biochem. 1970 Mar;3(1):33–43. [PubMed] [Google Scholar]
- Kim S., Nochumson S., Chin W., Paik W. K. A rapid method for the purification of S-adenosylmethionine: protein-carboxyl O-methyltransferase by affinity chromatography. Anal Biochem. 1978 Feb;84(2):415–422. doi: 10.1016/0003-2697(78)90059-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J., Stock J. Protein phosphatase 2A catalytic subunit is methyl-esterified at its carboxyl terminus by a novel methyltransferase. J Biol Chem. 1993 Sep 15;268(26):19192–19195. [PubMed] [Google Scholar]
- Lowenson J. D., Clarke S. Recognition of D-aspartyl residues in polypeptides by the erythrocyte L-isoaspartyl/D-aspartyl protein methyltransferase. Implications for the repair hypothesis. J Biol Chem. 1992 Mar 25;267(9):5985–5995. [PubMed] [Google Scholar]
- MacLaren D. C., Kagan R. M., Clarke S. Alternative splicing of the human isoaspartyl protein carboxyl methyltransferase RNA leads to the generation of a C-terminal -RDEL sequence in isozyme II. Biochem Biophys Res Commun. 1992 May 29;185(1):277–283. doi: 10.1016/s0006-291x(05)80987-8. [DOI] [PubMed] [Google Scholar]
- McFadden P. N., Clarke S. Methylation at D-aspartyl residues in erythrocytes: possible step in the repair of aged membrane proteins. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2460–2464. doi: 10.1073/pnas.79.8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobuchi M., Murao K., Takeda R., Kakimoto Y. Tissue-specific expression of isoaspartyl protein carboxyl methyltransferase gene in rat brain and testis. J Neurochem. 1994 Jan;62(1):322–328. doi: 10.1046/j.1471-4159.1994.62010322.x. [DOI] [PubMed] [Google Scholar]
- Mudgett M. B., Clarke S. Characterization of plant L-isoaspartyl methyltransferases that may be involved in seed survival: purification, cloning, and sequence analysis of the wheat germ enzyme. Biochemistry. 1993 Oct 19;32(41):11100–11111. doi: 10.1021/bi00092a020. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Gilman A. G. Synthetic peptide antisera with determined specificity for G protein alpha or beta subunits. Methods Enzymol. 1991;195:215–233. doi: 10.1016/0076-6879(91)95168-j. [DOI] [PubMed] [Google Scholar]
- Murray E. D., Jr, Clarke S. Metabolism of a synthetic L-isoaspartyl-containing hexapeptide in erythrocyte extracts. Enzymatic methyl esterification is followed by nonenzymatic succinimide formation. J Biol Chem. 1986 Jan 5;261(1):306–312. [PubMed] [Google Scholar]
- Murray E. D., Jr, Clarke S. Synthetic peptide substrates for the erythrocyte protein carboxyl methyltransferase. Detection of a new site of methylation at isomerized L-aspartyl residues. J Biol Chem. 1984 Sep 10;259(17):10722–10732. [PubMed] [Google Scholar]
- O'Connor C. M., Germain B. J., Guthrie K. M., Aswad D. W., Millette C. F. Protein carboxyl methyltransferase activity specific for age-modified aspartyl residues in mouse testes and ovaries: evidence for translation during spermiogenesis. Gamete Res. 1989 Mar;22(3):307–319. doi: 10.1002/mrd.1120220308. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M. Regulation and subcellular distribution of a protein methyltransferase and its damaged aspartyl substrate sites in developing Xenopus oocytes. J Biol Chem. 1987 Jul 25;262(21):10398–10403. [PubMed] [Google Scholar]
- Paik W. K., Kim S. Protein methylases during the development of rat brain. Biochim Biophys Acta. 1973 Jun 20;313(1):181–189. doi: 10.1016/0304-4165(73)90199-2. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Lee H. W., Lawson D. Age-dependency of various protein methylases. Exp Gerontol. 1971 Aug;6(4):271–277. doi: 10.1016/0531-5565(71)90008-8. [DOI] [PubMed] [Google Scholar]
- Potter S. M., Johnson B. A., Henschen A., Aswad D. W., Guzzetta A. W. The type II isoform of bovine brain protein L-isoaspartyl methyltransferase has an endoplasmic reticulum retention signal (...RDEL) at its C-terminus. Biochemistry. 1992 Jul 14;31(27):6339–6347. doi: 10.1021/bi00142a025. [DOI] [PubMed] [Google Scholar]
- Romanik E. A., Ladino C. A., Killoy L. C., D'Ardenne S. C., O'Connor C. M. Genomic organization and tissue expression of the murine gene encoding the protein beta-aspartate methyltransferase. Gene. 1992 Sep 10;118(2):217–222. doi: 10.1016/0378-1119(92)90191-q. [DOI] [PubMed] [Google Scholar]
- Saido T. C., Toyoshima S., Osawa T. Protein-O-carboxylmethyltransferase from cytosol and membranes of chicken erythrocytes. J Biochem. 1987 Aug;102(2):319–326. doi: 10.1093/oxfordjournals.jbchem.a122057. [DOI] [PubMed] [Google Scholar]
- Sato M., Yoshida T., Tuboi S. Primary structure of rat brain protein carboxyl methyltransferase deduced from cDNA sequence. Biochem Biophys Res Commun. 1989 May 30;161(1):342–347. doi: 10.1016/0006-291x(89)91602-1. [DOI] [PubMed] [Google Scholar]
- Solomon R., Katzir O., Egozi Y., Kloog Y. Identification of two distinct protein carboxyl methyltransferases in eucaryotic cells. FEBS Lett. 1988 Dec 5;241(1-2):131–135. doi: 10.1016/0014-5793(88)81045-7. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson R. C., Clarke S. Characterization of a rat liver protein carboxyl methyltransferase involved in the maturation of proteins with the -CXXX C-terminal sequence motif. J Biol Chem. 1992 Jul 5;267(19):13314–13319. [PubMed] [Google Scholar]
- Stephenson R. C., Clarke S. Identification of a C-terminal protein carboxyl methyltransferase in rat liver membranes utilizing a synthetic farnesyl cysteine-containing peptide substrate. J Biol Chem. 1990 Sep 25;265(27):16248–16254. [PubMed] [Google Scholar]
- Stock J. B., Lukat G. S., Stock A. M. Bacterial chemotaxis and the molecular logic of intracellular signal transduction networks. Annu Rev Biophys Biophys Chem. 1991;20:109–136. doi: 10.1146/annurev.bb.20.060191.000545. [DOI] [PubMed] [Google Scholar]
- Tejedor A., Noel J., Vinay P., Boulanger Y., Gougoux A. Characterization and metabolism of canine proximal tubules, thick ascending limbs, and collecting ducts in suspension. Can J Physiol Pharmacol. 1988 Aug;66(8):997–1009. doi: 10.1139/y88-164. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Clarke S. Methyl esterification of C-terminal leucine residues in cytosolic 36-kDa polypeptides of bovine brain. A novel eucaryotic protein carboxyl methylation reaction. J Biol Chem. 1993 Jun 25;268(18):13364–13371. [PubMed] [Google Scholar]
- Xie H., Clarke S. Protein phosphatase 2A is reversibly modified by methyl esterification at its C-terminal leucine residue in bovine brain. J Biol Chem. 1994 Jan 21;269(3):1981–1984. [PubMed] [Google Scholar]