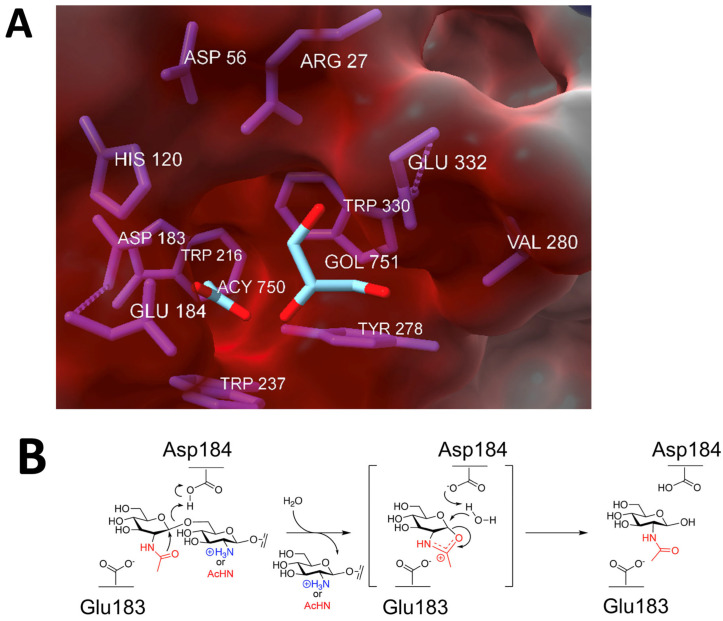
Figure 4.
Dispersin B’s active site and mechanism of action: (A) Electrostatic surface potential at the active site showing the negatively charged amino acids (Asp56, Asp183, Glu184, Glu332), which create a shallow anionic region in the catalytic pocket. The size of the pocket is approximately 12 Å. GOL, glycerol; ACY, acetate. Figure generated using ChimeraX [29]. (B) Substrate hydrolysis mechanism proposed for dispersin B and other glycoside hydrolase family 20 hexosaminidases. In this substrate-assisted mechanism, Glu184 acts as the acid/base. The nucleophile is the N-acetyl group of the substrate, which is assisted by Asp183. Both exo- (dPNAG) and endoglycosidic (PNAG) cleavage are shown, where the leaving group is either deacetylated or acetylated, respectively. A suitably positioned Asp183 helps stabilize the oxazolium ion in the transition state. Figure generated using ChemDraw (PerkinElmer).