Abstract
Stem-like CD8+ T cells (TSL) are a subset of immune cells with superior persistence and antitumor immunity. They are TCF1+ PD-1+ and important for the expansion of tumor specific CD8+ T cells in response to checkpoint blockade immunotherapy. In acute infections, naïve CD8+ T cells differentiate into effector and memory CD8+ T cells; in cancer and chronic infections, persistent antigen stimulation can lead to T cell exhaustion. Recent studies have highlighted the dichotomy between late dysfunctional (or exhausted) T cells (TLD) that are TCF1– PD-1+ and self-renewing TCF1+ PD-1+ TSL from which they derive. TCF1+ TSL cells are considered to have stem cell-like properties akin to memory T cell populations and can give rise to cytotoxic effector and transitory T cell phenotypes (TTE) which mediate tumor control. In this review, we will discuss recent advances made in research on the formation and expansion of TSL, as well as distinct niches required for their differentiation and maintenance in the setting of cancer. We will also discuss potential strategies to generate these cells, with clinical implications for stemness enhancement in vaccine design, immune checkpoint blockade (ICB), and adoptive T cell therapies.
Keywords: stem-like CD8 T cells (TSL), chronic viral infection, cancer models, immune, tertiary lymphoid structure (TLS), tumor microenvironment (TME)
1. Introduction
Immune checkpoint blockade (ICB) therapy has generated impressive success in recent years as 15~30% of cancer patients treated with ICB experience durable remissions (1). It has been proposed that ICB can reverse exhausted or late dysfunctional CD8+ T cells (TLD) to an effector-like state. However, recent studies have shown TLD cells have a terminally differentiated phenotype and may not be readily rescued. Rather, proliferative bursts of a relatively undifferentiated population of “stem-like” T cells (TSL) occur after ICB, which has been correlated with clinical benefit. These TSL are identified by their expression of transcription factor T cell factor-1 (TCF1), along with intermediate expression of inhibitory receptor, programmed cell death protein-1 (PD-1). TCF1+ PD-1+ TSL cells have the ability to expand, self-renew, and differentiate into transitory effector-like T cells (TTE) and TLD cells. TSL cells have been identified to play a vital role in sustaining the CD8+ T cell response in both chronic infection and cancer. Their presence is associated with positive clinical outcomes of checkpoint immunotherapies in patients with melanoma, colorectal, and non-small cell lung cancer (NSCLC) (2–4). Here we will review the latest developments regarding TSL population formation and expansion, along with the specific niches required for their maintenance and differentiation in the context of cancer. We will also explore potential approaches to produce TSL cells and discuss the therapeutic implications of enhancing stemness in adoptive T cell therapies, ICB, and vaccine design.
2. Formation, expansion, and hallmarks of stem-like CD8+ T cells
Stem-like CD8+ T cells have emerged as key players in response to ICB, as a subset of cells that retain stemness, have memory potential, and a high proliferative capacity. Targeting the PD-1: PD-L1 pathway with ICB treatment drives the expansion of these cells. This was first observed in chronic infection models (5–8) and subsequently in mouse and human cancers (2–4, 9, 10). As shown in Figure 1 , the proliferation burst encompasses not only expansion of TSL’S cells’ downstream TTE progeny, but also self-renewal of the TSL population. TSL self-propagate an epigenetically distinct, stable pool of TSL cells that persists during active disease. This population is armed for subsequent proliferative bursts and fuels a downstream differentiated effector population in an antigen-dependent manner. TSL cells survive and persist following antigen withdrawal, similar to conventional memory cells. Additionally, they can mount a recall response and continue to produce terminally differentiated progeny (11, 12). Although this subset is more proliferative than other differentiated exhausted subsets, compared to conventional memory cells, TSL have reduced proliferative capacity and cytokine function (13). TSL cells do share many markers with memory and naïve T cells ( Figure 2 ; Table 1 ). Markers such as CD62L and CD27 are more commonly expressed on naïve and memory populations, while CCR7 and CD28 are often expressed by both naïve and TSL cells. They are also induced/maintained by some similar transcription factors (TFs) including TCF1, BCL6, FOXO1, STAT3, JUN, MYB, BACH2, EOMES, TOX and ID3 (5–7, 14, 15). However, while TSL cells share many memory and stem-like features, they are committed to the exhaustion lineage, and transfer an exhausted phenotype to their progeny (16). While ICB treatment results in the expansion or proliferative bursts of this stem-like population, these cells and their effector progeny show distinct epigenetic features and metabolic state of exhausted T cells (17–19). Studies have observed that although commitment toward the T cell exhaustion phenotype begins as early as 5 days, it requires time for the epigenetic imprint to stabilize where it cannot be overcome by ICB (16, 20–22). The TF nuclear factor of activated T cells (NFAT) plays a pivotal role in effector and exhaustion responses of CD8+ T cells and induces the effector program with its associate TF activator protein 1 (AP-1) and its subunits JUN/FOS (23). In the absence of AP-1, NFAT induces a program of negative feedback leading to T cell exhaustion. Downstream targets of NFAT: TOX, NR4A1, NR4A2 are critical in enforcing T cell exhaustion in TSL cells (24–27). Absence of TOX results in the loss of the TSL population and loss over time in their effector progeny in chronic infection and tumor models (5, 7, 25, 26). Likewise, a recent study reported double deletion of NR4A1/NR4A2 in CD8+ tumor-infiltrating lymphocytes (TILs) resulted in murine tumor eradication after transfer as well as expansion of TSL population with increased chromatin accessibility of several stem-like/memory-related genes (28). TSL cells, however, do not express other co-inhibitory, exhausted T cell markers (TIM3, TIGIT, CTLA4) but do express low to intermediate levels of PD-1, not as a marker of exhaustion but rather activation (29). PD-1 has been shown to help preserve the co-expressing PD-1+ TCF1+ TSL population by attenuating TCR and co-stimulatory CD28, and by repressing downstream effector differentiation (22, 30, 31). TSL also express other markers such as inducible T cell costimulator (ICOS) molecule, CD28, CXCR5, SLAMF6 (also known as LY108), which denote a population of cells that have experienced antigen and require lymphoid homing (3–6, 14, 32). In chronic viral infection, TSL infiltrate B cell follicles correlating with CXCR5 expression on TSL whereas in tumors, SLAMF6 is highly expressed and positively correlates with TCF1 levels (2, 4, 9, 33).
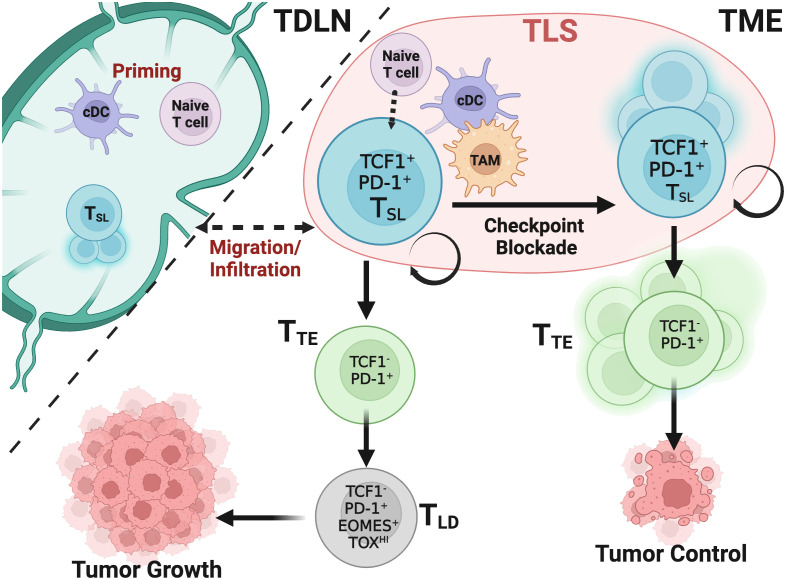
Figure 1.
TSL cells drive and maintain CD8+ T cell responses in cancer after ICB. Naïve and TSL CD8+ T cells are primed and activated in the tumor draining lymph node (TDLN) or tertiary lymphoid structures (TLS) within the tumor by conventional dendritic cells (cDCs) that present tumor derived antigen. A portion of these activated TSL cells reside in the TDLN and maintain a reservoir that migrate and infiltrate the tumor microenvironment (TME). Maintenance of TSL cells has yet to be fully determined within these immunological niches. Without ICB, following activation, TSL cells infiltrate tumors and rapidly undergo exhaustion in the presence of persistent antigen stimulation. While transitory effector CD8+ T cells (TTE) cells differentiate from TSL cells, TTE quickly adopt a late dysfunctional (TLD) phenotype but can carry a level of some tumor control through cytotoxic cytokines and tumor cell targeting. Upon ICB, the TSL population undergoes self-renewal and proliferation, giving rise to the TTE subset and this supports the majority of the CD8+ T cell antitumoral response, leading to tumor control.
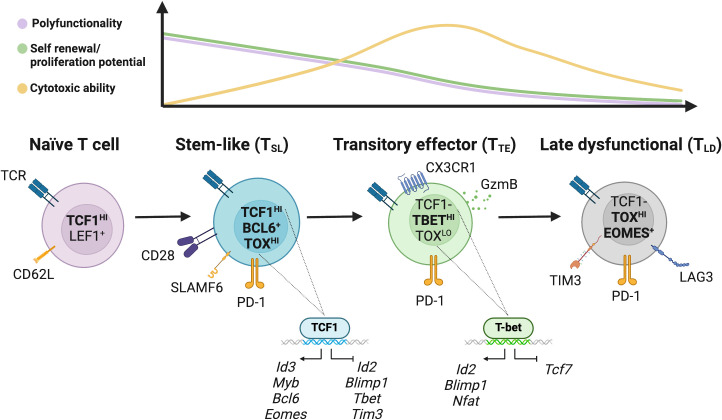
Figure 2.
Model of the characteristics and differentiation of CD8+ T cell states in cancer. The transcription factors TCF1 and LEF1, as well as the adhesion and lymphocyte homing molecules CD62L, are highly expressed in naïve T cells. Downstream, the population of stem-like CD8+ T (TSL) cells with strong polyfunctionality and self-renewal ability is defined by TCF1. These cells have a strong proliferation capacity, are primarily quiescent in vivo, and are able to support the CD8+ T cell response. By suppressing the expression of effector-associated genes like Id2, Blimp-1, Tbet and Tbx21 and stimulating memory-associated genes like Eomes, Myb, Bcl-6, TCF1 facilitates the generation, maintenance, and functionality of these cells. Phenotypically, TSL cells display CD28 and ICOS costimulatory markers, low or moderate amounts of PD-1, SLAMF6, and CXCR5. TSL cells expand upon ICB and both maintain the TSL reservoir and differentiate into further subpopulations. Differentiation of TSL give rise to downstream transitory effector CD8+ T cells (TTE) that express high PD-1 receptor, proliferate rapidly in steady state down regulating TCF1 expression, and upregulate T-BET. T-BET inhibits TOX-mediated development of late dysfunctional T cells (TLD) phenotype in TTE cells. Transitory cells exhibit the expression of CX3 chemokine receptor 1 (CX3CR1) and PD-1. TTE proliferate to help target and eliminate tumor cells. After chronic antigen stimulation, these TTE cells develop into TLD cells which are characterized by high expression of checkpoint receptors (PD-1, TIM3, LAG3, CTLA-4, TIGIT, and CD101), poor polyfunctionality, low proliferation capacity, but retain some cytotoxic potential.
Table 1.
Summary of the transcription factors, biomarkers, and key features that define CD8+ T cell subsets in cancer.
| Murine■ Human■ Both■ |
Naïve T cell | T Stem-like (TSL) |
Transitory Effector (TTE) |
Late Dysfunctional (TLD) |
|---|---|---|---|---|
| Transcription Factors | TCF1, LEF1 | TCF1, LEF1, EOMES, TOX, MYB, FOXO1, JUN, STAT3 ID3, BCL6, BACH2, EGR2 |
TBET, BLIMP1, BATF, IRF4, ID2, NFAT, RUNX3, NR4A | EOMES, TOX, BATF, NFAT |
| Biomarkers | CD62L CCR7 CD28 CD27 CD45RA CD45 |
TCF1 PD-1 LY108/SLAMF6 CXCR5 CD28 ICOS CCR7 CD69 CD45RO |
PD-1 GZMB TBET LAG3 CX3CR1 CD45RO |
PD-1 TIM3 LAG3 TIGIT CD101 CTLA4 CX3CR1 CD45RO |
| Key Features | Immature cell Circulate in lymph and blood Feeds downstream subsets |
Self-renewal Expands/proliferate after ICB Persistent population pool Feeds downstream effector subsets |
Effector/cytotoxic killing to control tumor growth | Increased expression of inhibitory receptors Limited killing capacity and proliferation |
All figures created with BioRender.com.
Another critical feature of TSL cells is the uniform expression of TCF1, encoded by the Tcf7 gene, which is essential for the formation and function of this population (3, 5–7, 10, 14). Originally identified as a TF essential for thymocyte development, both TCF1 and its homologue LEF1, are now known to promote memory T cell differentiation and inhibit effector differentiation (34, 35). Open chromatin sites in TSL cells are highly enriched in the TCF/LEF motif, similar to naïve T cells, and overlap frequently with TCF1 binding peaks, suggesting direct regulation by TCF1 (20, 35, 36). Studies in chronic infection and tumor models have shown that loss of TCF1 in CD8+ T cells limits their maintenance, function, and overall response to ICB, but does not diminish their overall function (3, 7, 10, 37). Additionally, a preclinical tumor study showing ectopic expression of TCF1 skews TILs to adopt a TSL phenotype while enhancing their polyfunctionality and further suppressing inhibitory receptors and modulating the transcriptome to further suppress TFs like BLIMP1, RUNX3, and TOX to improve viral and tumor control (38). A recent study disputes that tumor immunogenicity dictates reliance on TCF1 for ICB efficacy (39). However, antitumor responses in poorly immunogenic tumors can be improved by optimizing T cell priming through either vaccination or enhancing antigen presentation on tumors (39). Additionally, frequency of TCF7-expressing CD8+ T cells in melanoma can correlate to positive response to ICB, whereas in advanced clear cell renal carcinoma patients, it failed to predict any clinical outcomes (40, 41). How TCF1 directly aids in forming and expanding this crucial stem-like population within its environment is still debated.
Together, the key features that define the formation and expansion of TSL cells encompass multiple regulatory pathways. Many of the features of TSL are similar to other well defined T cell subsets, therefore it is crucial to establish how regulatory mechanisms operate uniquely in the TSL population in a variety of environments. We have described how TSL cells self-renew while maintaining an exhausted lineage; next we will delve into how this subset continues to feed into the pool of CD8+ T cells and help sustain responses to ICB.
3. Differentiation and maintenance of stem-like CD8+ T cells
Studies from chronic viral infection and tumor models have characterized two populations of epigenetically and spatially distinct populations of CD8+ T cells: TCF1+ PD-1+ TIM3- CD8+ TSL and their progeny, TCF1- PD-1+ CD8+ T transitory effector-like CD8+ T cells (TTE) (3–7, 9, 10). The TTE cells become terminally differentiated, late dysfunctional TCF1lo/- PD-1+ TIM3+ T cells (TLD) that carry distinct transcriptional and epigenetic programs that differ from those seen in traditional memory and effector populations, both in cancer and chronic viral infection ( Figures 1 , 2 ; Table 1 ) (8, 19, 22). It has been shown that TSL drive the proliferative response after immunotherapy and are often associated with clinical benefit, while TLD populations have limited survival and re-expansion potential (3–7, 10, 42). TSL cells and their progeny are committed to an exhausted phenotype, however a unique feature of the TSL population being its ability to be stimulated to expand by ICB, whereas TLD cells cannot be reinvigorated (5, 7, 8, 16, 43). On the other hand, the majority of the tumor specific population exhibits a TLD phenotype, which may indicate a continuous immune response that requires a precursor population generating and infiltrating from external locations (37, 44–49).
The generation and maintenance of TSL cells may be significantly impacted by varying environmental cues. In chronic infection, most TSL cells are located within B cell follicles and the T cell zone of the spleen while their progeny exist within the red pulp taking up residency rather than migration (6, 14, 50). Contrastingly in tumors, TSL cells migrate between perivascular niches or tertiary lymphoid structures (TLS) within the tumor and reservoirs in the tumor-draining lymph node (TDLN) ( Figure 1 ) (3, 9, 32, 51–61). Blocking migration using sphingosine 1-phosphate receptor 1 (S1P1)-agonist FTY720 in multiple preclinical tumor models prevented tumor regression and challenged the understanding that anti-PD-1 immunotherapy primarily targets intratumoral T cells. This also suggests that TSL migration to TDLN may even be required for TSL cell maintenance (51, 52, 55, 60, 61). These specific tissue niches likely have two purposes for maintaining TSL cells: to sequester away this population from inflammatory cues that quickly drive differentiation into exhausted phenotypes and to provide close, tightly regulated contact with antigen-presenting cells (APCs) such as dendritic cells (DCs) (60, 62). Recent preclinical research also implies that molecularly distinct lymph-node resident CD8+ memory-like and TSL cells are sole mediators of ICB (61, 63). Two additional recent studies in non-small cell lung cancer (NSCLC) and head and neck squamous cell carcinoma (HNSCC) respectively, also support the idea that TSL cells respond to immunotherapy within the lymph nodes (64, 65). Clusters of TSL populations and APCs are also linked to significant T cell infiltration in human malignancies, whereas their absence may lead to immune evasion (9, 34). While TSL cells are clustered with APCs and even CD4+ T cells within TLSs creating a supportive network to promote effective differentiation into TTE subsets, TLD cells are more scattered throughout the tumor parenchyma where they can readily engage with target cells (57, 66–68). Evidence also suggests that immunotherapy responses in sarcoma, melanoma and renal cell carcinoma are favorably linked with TLSs containing B cells and TSL cells (33, 69, 70). In tumors that possess obstacles preventing the infiltration of T cells, such as solid tumors, immune cell niches can persist and harbor concentrated populations of TSL cells that are aggregated with APCs (71). Cells in such niches were able to rapidly regenerate the immune response in patients with brain metastases and these immune niches were prognostic for local disease control (71). Thus, it is likely the interactions of TSL cells with DCs and B cells within these niches are influential in the maintenance and function of TSL and are required for durable CD8+ T cell responses. As previously mentioned, epigenetic analysis of TSL in chronic infection compared to TLD revealed unique open chromatin sites, and TSL subsets show increased accessibility to XCL1 which is involved in the interactions between DCs and T cells (3, 4, 36, 72). XCL1 expressed on T cells promotes the recruitment of XCR1+ conventional type 1 DCs (cDC1) which have superior antigen processing and cross-presentation capabilities (73). Several groups have highlighted the necessity of cDC1s in sustaining TSL cells and inducing the proliferative burst after ICB within the TLS as well as in maintaining the TDLN TSL reservoir in preclinical tumor and chronic infection models, and patient samples (60, 62, 74). Additionally, the B7/CD28 pathway, expressed on DCs and T cells respectively, may have a role in structuring how these interactions sustain the immune response as TSL have high CD28 expression that is necessary for the proliferative burst after ICB (75, 76). By blocking B7 costimulatory molecule on APCs or deletion of CD28 on T cells, effective responses to PD-1/PD-L1 ICB were diminished (76).
Another environmental cue being investigated is the CXCR3 pathway as a significant axis of immunotherapy response that regulates the infiltration and spatial positioning of T cells near APCs expressing the ligands CXCL9/10/11 within the murine and human tumor microenvironment (TME) (54, 77, 78). As multiple myeloid populations within the TME express the ligand CXCL9, including both DCs and tumor-associated macrophages (TAMs), and these chemokines are broadly induced in response to treatment, it remains another avenue to investigate in the maintenance of TSL within TLSs (79–82). In the TME, macrophages are more abundant and express higher levels of CXCL9 than compared to DCs and may play a more prominent role in the TME compared to DCs in the TDLN (81).
Differentiation of TSL into their cytolytic progeny TTE cells has proved vital to the efficacy of ICB. The maintenance of this population via the TDLN reservoir or in TLSs within the tumor additionally have gained recognition in contributing to improved clinical outcomes. Many of the networks and signaling pathways involved in these environments will likely aid in determining future successes of therapeutics.
4. Therapeutic potential of stem-like CD8+ T cells in cancer
4.1. Immune checkpoint blockade
ICB therapy against inhibitory receptors PD-1 and CTLA4 of TILs has shown success in mounting a T cell response against tumors in many cancer types. Efficacy is highest in tumors with more mutational burden and typically higher TIL infiltration suggesting leveraging an already present immune response. Prior to the role of TSL, it was thought that TLD being “rescued” from their late dysfunctional phenotype to a less exhausted, more effector phenotype was the primary mechanism of ICB (83). Some clinical studies have shown an abundance of cells with a TTE or TLD phenotype rather than TSL cells can provide a better predictor for response to ICB (84–88). While TCF1+ expression by TILs in human melanoma coincides with clinical benefit of ICB, TCF1 is produced also by bystander TILs which are less relevant for antitumor response. High frequencies of TCF1+ PD-1+ TSL thus may be an unreliable biomarker as a portion of these cells are not tumor-specific (40, 89). Likely, the ratio of TSL to more differentiated TILs may represent a more suitable biomarker for outcome prediction as TSL frequencies are comparable to those observed in responders versus non-responders (4, 40). In chronic infection and tumor models, TSL have been shown to be critical in amplifying the response to ICB by self-renewal, expansion, and differentiation into TTE, supplying the pool of cytotoxic cells and mediating disease control (90). Given their crucial role in ICB, it is imperative to effectively control TSL cells. Continuous driving of differentiation by immune checkpoints can negatively impact maintenance of TSL cells and ultimately result in loss of the ability to expand and differentiate, driving patients toward a refractory state (22, 91, 92). Bi-specific antibody therapy has shown promising outcomes in patients with hematologic malignancies, although in cancers more resistant to ICB and favorable outcomes are limited. One drug construct uses an anti-PD-1 molecule as a targeting moiety fused to a stimulatory IL-2 variant (IL-2v) to deliver IL-2 to PD-1+ T cells in the TME. Combining with anti-PD-L1 treatment resulted in murine tumor regression, enhanced infiltration of the TSL population, and reprogramming of TAMs (93). It is important to note, prolonged exposure of T cells to bispecifics through continuous infusion can also cause cells to adopt the TLD phenotype and therefore must be carefully evaluated (93, 94). Other therapeutic strategies taken to clinical trial include inhibiting cell division, T cell receptor (TCR) signaling, or epigenetic pathways to hinder TSL differentiation (18, 19, 95–97). Additionally, depleting or altering T cell signaling pathways in TSL cells have shown to promote stem-like phenotype retention, allowing these cells to persist in harsh environments that would otherwise push these populations towards TLD phenotype, and instead still produce effective TTE progeny (98–100). Clinical data has also shown that ICB therapy induced expansion of pre-treatment TSL cells present in patients who were responders compared to non-responders which had more pre-treatment TLD phenotypes, experienced therapy resistance (10, 40, 48, 49, 59, 90).
Quantity or presence of TSL alone may be insufficient as a marker of response, because as previously mentioned, APC-dense niches or TLSs tolerant for TSL self-renewal or expansion, may additionally be required for effective responses. Clinical observations have revealed that tumors with such regions correlate with favorable therapeutic responses (51, 59, 71, 87, 88). Additionally in other preclinical studies it has been observed that blocking T cell egress from TDLN, surgically removing the TDLN, or disrupting the migration of T cells from the TME diminishes ICB response (51–54, 61). Further new studies from patient samples of NSCLC, HNSCC, and melanoma also indicate TSL cells respond to ICB directly in the TDLN, displaying local clonal expansion and subsequent migration of these new clones to the TME (44, 64, 65, 83, 90). Therefore, targeting the establishment and cultivation of these regions within the TME or TDLN, to enhance TSL maintenance and differentiation, could further increase efficacy (101, 102).
4.2. Adoptive cellular therapy
This therapy encompasses two main approaches: ex vivo expansion of TILs or genetic modification of peripheral blood mononuclear cells (PBMC)-derived T cells for tumor specific subsets and subsequent reintroduction into the patient. Ex vivo manufacturing and expansion strategies to induce TSL cells include introducing IL-7, IL-15, and IL-21 to promote expression of associated genes like TCF7, Eomes, and Bcl6 (103–107), or promoting Notch signaling upstream of TCF1 (108, 109). Suppressing genes associated with late dysfunctional or exhaustive phenotypes such as Tbet, BATF, EOMES pharmacologically ex vivo can maintain stem-like genes (TCF1/LEF1) and retains TSL cell polyfunctionality (110, 111). Numerous studies of both preclinical models and patients of ACT observe that less differentiated, memory and stem-like cells elicit more of an effective antitumoral response (112–118). Genetically engineering T cells using retroviral transduction to incorporate a tumor reactive TCR or a chimeric antigen receptor (CAR-T) has become standard of care for many hematologic malignancies (119–125). Increased populations of terminally exhausted CD8+ CAR-T cells present in pre-treatment product correlate with worse outcomes however, presence of more naïve and memory-like CAR-T phenotypes are correlated with increased response rates (126–128). Although extensive clinical research into TSL phenotypes in CAR-T products has yet to be conducted, one recent study identified that PD-1+ TCF1+ stem-like CAR-T and PD-1+ TIM3+ effector-like CAR-T correlated with improved clinical outcomes (129). This study highlights the importance of PD-1 expression on CAR-T cells post-infusion as a marker of activation rather than exhaustion for optimal activation as well as the potential for optimizing stem-like phenotypes in CAR-T subsets to potentially improve clinical outcomes.
Study of the epigenetic landscape of TSL, TTE and TLD subsets has revealed several targets for controlling the differentiation and antitumor response and are now in preclinical CAR-T models (130–133). Exploration of the chromatin accessibility of CAR-T cells at the single cell level, both in vitro and in vivo, identified two distinct subsets (133). The subsets consisted of intermediate exhausted CAR-T cells enriched for TFs of TSL cells (JUN/FOS) and another with enriched motifs of BATF and IRF4 resembling terminally exhausted or the TLD CAR-T subset. CAR-T cells with knockdown of BATF, IRF4 or NR4A expression had enhanced effector function, inhibited exhaustion and prolonged CAR-T cell persistence in vivo (133, 134). A dual knockout of genes PRDM1 (encoding BLIMP1 TF) and NR43A in preclinical murine models, skewed CAR-T cell phenotypes toward TSL subsets and away from TLD, improving antitumor responses and not achieved by single knockouts (132).
Additionally, several preclinical CAR-T models targeting overexpression of TFs specific for TSL such as c-Jun and FOXO1, promote stem-like phenotypes, enhanced expansion potential, persistence and therapeutic efficacy in vivo (130, 131). Other factors such as hub transcription factors, like FOXP1 and KLF2 that have high numbers of enhancers that are positioned in the center of gene regulatory networks, can serve as checkpoints that control lineage-defining TFs between stem-like and effector CAR-T, and the decision between effector and late dysfunctional CAR-T cells, respectively (135). While harnessing the power of TSL cell phenotype in CAR-T therapy by targeting key transcriptional regulators may lead to further successful trials, investigating the relationships of other immune cells or combination therapy in altering other environmental cues could be crucial to their advancement.
Pre-existing TLS or APC-dense niches may also be required for generating stem-like CAR-T phenotypes and catering to the cultivation of these environments may also increase their persistence (63, 136, 137). Utilizing stem-like CD8+ T cells and their respective molecular determinants as biomarkers of response to CAR-T may also prove beneficial within a clinical setting.
Cancer immunotherapy such as ICB and CAR-T rely on T cell infiltration. The accumulated evidence above shows that combining multiple therapeutic agents is crucial for cancer immunotherapy and targeting stem-like CD8+ T cells requires more than one approach.
4.3. Cancer vaccination
Studies in the forefront of cancer vaccination are seeking to harness the self-renewal, long lasting durability, and sustainability of the TSL subset by targeting common tumor antigens or patient specific neoantigens (neoAg) (138–140). Recent advances in genomic sequencing have led to personalized cancer vaccines targeting neoAg. Early studies show feasibility in mice and clinical trials, but neoAg targeted CD8+ T cell responses have been limited (139–145). Coupling self-assembling nanoparticle vaccine platform technology, exploiting its ability to quickly drain via lymphatics to DCs and enhance antigen presentation to CD8s, the SNP-7/8a intravenous vaccination generated more TSL cells that are receptive to ICB in a therapeutic murine model (146). Additionally, adenovirus (Ad)-vectored vaccines encoding tumor neoAg combined with ICB have been shown to eradicate large tumors and increases in TSL cells in the TDLN and TTE cells within the TME in mice and have translated into similar results within the clinic (147). Further, studies harnessing not only TSL cells but also other tumor targeting progenitors, like stem-like natural killer (NK) cells are gaining interest. Introduced at the contraction phase after immunization with an artificial adjuvant vector cell (aAVC), an IL-2/anti-IL-2 monoclonal antibody complex (IL-2Cx) combination activated stem-like subsets that correlated with therapeutic responses, and induced long-term memory CD8+ T cells that conferred protection against tumor rechallenge in a leukemic model (148). While tumor vaccine trial successes have been mixed, expanding the population of tumor specific TSL cells is likely the key consideration for the future of favorable tumor vaccine outcomes.
5. Conclusions and outlook
The role of stem-like T cells has been underscored in recent studies, highlighting their potential to improve the antitumor effect of immunotherapies. However, to fully exploit this potential, a complete understanding of how TSL cells form, maintain, and function is necessary. Recent advances in deciphering this subsets’ key characteristics and hallmarks have led even further to questions that require investigation. The most vital questions and potential targets will likely center around TSL and APC interactions within their relevant niches in a variety of models. The targeting and harnessing of TSL cells will require multiple points of application.
In conclusion, while significant strides have been made in understanding the role and potential of TSL cells in cancer therapy, there is still much work to be done. Future research should focus on elucidating the regulatory circuits that control these cells, understanding the APC interactions with intratumoral TSL cells and within niches, and developing methods for TSL cell generation. These efforts will be crucial in harnessing TSL cells for therapeutic interventions and enhancing immunotherapy against cancer. The exploration of combination therapies and strategies to maintain the “stemness” of T cells represent promising avenues for future research and could revolutionize cancer treatment.
Acknowledgments
All figures created with BioRender.com.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded, in part, by NIH grants R01 CA193167 (to YY), R01 HL151195 (to YY and XH), R01 CA260858 (to YY and XH).
Author contributions
CS: Conceptualization, Writing – original draft. ND: Writing – review & editing. XH: Conceptualization, Supervision, Writing – review & editing. YY: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Sharma P, Allison JP. Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol. (2020) 20:75–6. doi: 10.1038/s41577-020-0275-8 [DOI] [PubMed] [Google Scholar]
- 2. Brummelman J, Mazza EMC, Alvisi G, Colombo FS, Grilli A, Mikulak J, et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8(+) T cells infiltrating human tumors. J Exp Med. (2018) 215:2520–35. doi: 10.1084/jem.20180684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. (2019) 50:195–211 e110. doi: 10.1016/j.immuni.2018.12.021 [DOI] [PubMed] [Google Scholar]
- 4. Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. (2019) 20:326–36. doi: 10.1038/s41590-019-0312-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. (2016) 537:417–21. doi: 10.1038/nature19330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He R, Hou S, Liu C, Zhang A, Bai Q, Han M, et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. (2016) 537:412–28. doi: 10.1038/nature19317 [DOI] [PubMed] [Google Scholar]
- 7. Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, et al. T cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity. (2016) 45:415–27. doi: 10.1016/j.immuni.2016.07.021 [DOI] [PubMed] [Google Scholar]
- 8. Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, et al. The epigenetic landscape of T cell exhaustion. Science. (2016) 354:1165–9. doi: 10.1126/science.aae0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. (2019) 576:465–70. doi: 10.1038/s41586-019-1836-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1(-)CD8(+) tumor-infiltrating T cells. Immunity. (2019) 50:181–194 e186. doi: 10.1016/j.immuni.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wieland D, Kemming J, Schuch A, Emmerich F, Knolle P, Neumann-Haefelin C, et al. TCF1(+) hepatitis C virus-specific CD8(+) T cells are maintained after cessation of chronic antigen stimulation. Nat Commun. (2017) 8:15050. doi: 10.1038/ncomms15050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tonnerre P, Wolski D, Subudhi S, Aljabban J, Hoogeveen RC, Damasio M, et al. Differentiation of exhausted CD8(+) T cells after termination of chronic antigen stimulation stops short of achieving functional T cell memory. Nat Immunol. (2021) 22:1030–41. doi: 10.1038/s41590-021-00982-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beltra JC, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, et al. Developmental relationships of four exhausted CD8(+) T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity. (2020) 52:825–841 e828. doi: 10.1016/j.immuni.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. (2016) 17:1187–96. doi: 10.1038/ni.3543 [DOI] [PubMed] [Google Scholar]
- 15. Petrovas C, Ferrando-Martinez S, Gerner MY, Casazza JP, Pegu A, Deleage C, et al. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci Transl Med. (2017) 9. doi: 10.1126/scitranslmed.aag2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. (2016) 354:1160–5. doi: 10.1126/science.aaf2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo Y, Xie YQ, Gao M, Zhao Y, Franco F, Wenes M, et al. Metabolic reprogramming of terminally exhausted CD8(+) T cells by IL-10 enhances anti-tumor immunity. Nat Immunol. (2021) 22:746–56. doi: 10.1038/s41590-021-00940-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gabriel SS, Tsui C, Chisanga D, Weber F, Llano-Leon M, Gubser PM, et al. Transforming growth factor-beta-regulated mTOR activity preserves cellular metabolism to maintain long-term T cell responses in chronic infection. Immunity. (2021) 54:1698–1714 e1695. doi: 10.1016/j.immuni.2021.06.007 [DOI] [PubMed] [Google Scholar]
- 19. Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell. (2017) 170:142–157 e119. doi: 10.1016/j.cell.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, et al. Chromatin states define tumor-specific T cell dysfunction and reprogramming. Nature. (2017) 545:452–6. doi: 10.1038/nature22367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Utzschneider DT, Gabriel SS, Chisanga D, Gloury R, Gubser PM, Vasanthakumar A, et al. Early precursor T cells establish and propagate T cell exhaustion in chronic infection. Nat Immunol. (2020) 21:1256–66. doi: 10.1038/s41590-020-0760-z [DOI] [PubMed] [Google Scholar]
- 22. Chen Z, Ji Z, Ngiow SF, Manne S, Cai Z, Huang AC, et al. TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity. (2019) 51:840–855 e845. doi: 10.1016/j.immuni.2019.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez GJ, Pereira RM, Aijo T, Kim EY, Marangoni F, Pipkin ME, et al. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity. (2015) 42:265–78. doi: 10.1016/j.immuni.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. (2019) 571:265–9. doi: 10.1038/s41586-019-1326-9 [DOI] [PubMed] [Google Scholar]
- 25. Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. (2019) 571:211–8. doi: 10.1038/s41586-019-1325-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yao C, Sun HW, Lacey NE, Ji Y, Moseman EA, Shih HY, et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8(+) T cell persistence in chronic infection. Nat Immunol. (2019) 20:890–901. doi: 10.1038/s41590-019-0403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seo H, Chen J, Gonzalez-Avalos E, Samaniego-Castruita D, Das A, Wang YH, et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8(+) T cell exhaustion. Proc Natl Acad Sci USA. (2019) 116:12410–5. doi: 10.1073/pnas.1905675116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Srirat T, Hayakawa T, Mise-Omata S, Nakagawara K, Ando M, Shichino S, et al. NR4a1/2 deletion promotes accumulation of TCF1(+) stem-like precursors of exhausted CD8(+) T cells in the tumor microenvironment. Cell Rep. (2024) 43:113898. doi: 10.1016/j.celrep.2024.113898 [DOI] [PubMed] [Google Scholar]
- 29. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. (2018) 18:153–67. doi: 10.1038/nri.2017.108 [DOI] [PubMed] [Google Scholar]
- 30. Burger ML, Cruz AM, Crossland GE, Gaglia G, Ritch CC, Blatt SE, et al. Antigen dominance hierarchies shape TCF1(+) progenitor CD8 T cell phenotypes in tumors. Cell. (2021) 184:4996–5014 e4926. doi: 10.1016/j.cell.2021.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shakiba M, Zumbo P, Espinosa-Carrasco G, Menocal L, Dundar F, Carson SE, et al. TCR signal strength defines distinct mechanisms of T cell dysfunction and cancer evasion. J Exp Med. (2022) 219(2). doi: 10.1084/jem.20201966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Connolly KA, Kuchroo M, Venkat A, Khatun A, Wang J, William I, et al. A reservoir of stem-like CD8(+) T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci Immunol. (2021) 6:eabg7836. doi: 10.1126/sciimmunol.abg7836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. (2020) 577:561–5. doi: 10.1038/s41586-019-1914-8 [DOI] [PubMed] [Google Scholar]
- 34. Escobar G, Mangani D, Anderson AC. T cell factor 1: A master regulator of the T cell response in disease. Sci Immunol. (2020) 5(53). doi: 10.1126/sciimmunol.abb9726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao X, Shan Q, Xue HH. TCF1 in T cell immunity: a broadened frontier. Nat Rev Immunol. (2022) 22:147–57. doi: 10.1038/s41577-021-00563-6 [DOI] [PubMed] [Google Scholar]
- 36. Jadhav RR, Im SJ, Hu B, Hashimoto M, Li P, Lin JX, et al. Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proc Natl Acad Sci USA. (2019) 116:14113–8. doi: 10.1073/pnas.1903520116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zehn D, Thimme R, Lugli E, de Almeida GP, Oxenius A. ‘Stem-like’ precursors are the fount to sustain persistent CD8(+) T cell responses. Nat Immunol. (2022) 23:836–47. doi: 10.1038/s41590-022-01219-w [DOI] [PubMed] [Google Scholar]
- 38. Shan Q, Hu S, Chen X, Danahy DB, Badovinac VP, Zang C, et al. Ectopic Tcf1 expression instills a stem-like program in exhausted CD8(+) T cells to enhance viral and tumor immunity. Cell Mol Immunol. (2021) 18:1262–77. doi: 10.1038/s41423-020-0436-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Escobar G, Tooley K, Oliveras JP, Huang L, Cheng H, Bookstaver ML, et al. Tumor immunogenicity dictates reliance on TCF1 in CD8(+) T cells for response to immunotherapy. Cancer Cell. (2023) 41:1662–1679 e1667. doi: 10.1016/j.ccell.2023.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. (2018) 175:998–1013 e1020. doi: 10.1016/j.cell.2018.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ficial M, Jegede OA, Sant’Angelo M, Hou Y, Flaifel A, Pignon JC, et al. Expression of T-cell exhaustion molecules and human endogenous retroviruses as predictive biomarkers for response to nivolumab in metastatic clear cell renal cell carcinoma. Clin Cancer Res. (2021) 27:1371–80. doi: 10.1158/1078-0432.CCR-20-3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci USA. (2008) 105:15016–21. doi: 10.1073/pnas.0801497105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Philip M, Schietinger A. CD8(+) T cell differentiation and dysfunction in cancer. Nat Rev Immunol. (2022) 22:209–23. doi: 10.1038/s41577-021-00574-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yost KE, Chang HY, Satpathy AT. Recruiting T cells in cancer immunotherapy. Science. (2021) 372:130–1. doi: 10.1126/science.abd1329 [DOI] [PubMed] [Google Scholar]
- 45. Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. (2018) 9:2724. doi: 10.1038/s41467-018-05072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li H, van der Leun AM, Yofe I, Lubling Y, Gelbard-Solodkin D, van Akkooi ACJ, et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell. (2019) 176:775–789 e718. doi: 10.1016/j.cell.2018.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumor infiltrates. Nature. (2018) 557:575–9. doi: 10.1038/s41586-018-0130-2 [DOI] [PubMed] [Google Scholar]
- 48. Oliveira G, Stromhaug K, Klaeger S, Kula T, Frederick DT, Le PM, et al. Phenotype, specificity and avidity of antitumor CD8(+) T cells in melanoma. Nature. (2021) 596:119–25. doi: 10.1038/s41586-021-03704-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caushi JX, Zhang J, Ji Z, Vaghasia A, Zhang B, Hsiue EH, et al. Author Correction: Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature. (2021) 598:E1. doi: 10.1038/s41586-021-03893-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Im SJ, Konieczny BT, Hudson WH, Masopust D, Ahmed R. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proc Natl Acad Sci USA. (2020) 117:4292–9. doi: 10.1073/pnas.1917298117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dammeijer F, van Gulijk M, Mulder EE, Lukkes M, Klaase L, van den Bosch T, et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell. (2020) 38:685–700 e688. doi: 10.1016/j.ccell.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 52. Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, et al. Systemic immunity is required for effective cancer immunotherapy. Cell. (2017) 168:487–502 e415. doi: 10.1016/j.cell.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fransen MF, Schoonderwoerd M, Knopf P, Camps MG, Hawinkels LJ, Kneilling M, et al. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight. (2018) 3(23). doi: 10.1172/jci.insight.124507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, et al. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity. (2019) 50:1498–1512 e1495. doi: 10.1016/j.immuni.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li Z, Tuong ZK, Dean I, Willis C, Gaspal F, Fiancette R, et al. In vivo labeling reveals continuous trafficking of TCF-1+ T cells between tumor and lymphoid tissue. J Exp Med. (2022) 219(6). doi: 10.1084/jem.20210749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prokhnevska N, Cardenas MA, Valanparambil RM, Sobierajska E, Barwick BG, Jansen C, et al. CD8(+) T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor. Immunity. (2023) 56:107–124 e105. doi: 10.1016/j.immuni.2022.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hua Y, Vella G, Rambow F, Allen E, Antoranz Martinez A, Duhamel M, et al. Cancer immunotherapies transition endothelial cells into HEVs that generate TCF1(+) T lymphocyte niches through a feed-forward loop. Cancer Cell. (2022) 40:1600–1618 e1610. doi: 10.1016/j.ccell.2022.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoch T, Schulz D, Eling N, Gomez JM, Levesque MP, Bodenmiller B. Multiplexed imaging mass cytometry of the chemokine milieus in melanoma characterizes features of the response to immunotherapy. Sci Immunol. (2022) 7:eabk1692. doi: 10.1126/sciimmunol.abk1692 [DOI] [PubMed] [Google Scholar]
- 59. Magen A, Hamon P, Fiaschi N, Soong BY, Park MD, Mattiuz R, et al. Intratumoral dendritic cell-CD4(+) T helper cell niches enable CD8(+) T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat Med. (2023) 29:1389–99. doi: 10.1038/s41591-023-02345-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schenkel JM, Herbst RH, Canner D, Li A, Hillman M, Shanahan SL, et al. Conventional type I dendritic cells maintain a reservoir of proliferative tumor-antigen specific TCF-1(+) CD8(+) T cells in tumor-draining lymph nodes. Immunity. (2021) 54:2338–2353 e2336. doi: 10.1016/j.immuni.2021.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huang Q, Wu X, Wang Z, Chen X, Wang L, Lu Y, et al. The primordial differentiation of tumor-specific memory CD8(+) T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell. (2022) 185:4049–66.e4025. doi: 10.1016/j.cell.2022.09.020 [DOI] [PubMed] [Google Scholar]
- 62. Dahling S, Mansilla AM, Knopper K, Grafen A, Utzschneider DT, Ugur M, et al. Type 1 conventional dendritic cells maintain and guide the differentiation of precursors of exhausted T cells in distinct cellular niches. Immunity. (2022) 55:656–670 e658. doi: 10.1016/j.immuni.2022.03.006 [DOI] [PubMed] [Google Scholar]
- 63. Gebhardt T, Park SL, Parish IA. Stem-like exhausted and memory CD8(+) T cells in cancer. Nat Rev Cancer. (2023) 23:780–98. doi: 10.1038/s41568-023-00615-0 [DOI] [PubMed] [Google Scholar]
- 64. Pai JA, Hellmann MD, Sauter JL, Mattar M, Rizvi H, Woo HJ, et al. Lineage tracing reveals clonal progenitors and long-term persistence of tumor-specific T cells during immune checkpoint blockade. Cancer Cell. (2023) 41:776–790 e777. doi: 10.1016/j.ccell.2023.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rahim MK, Okholm TLH, Jones KB, McCarthy EE, Liu CC, Yee JL, et al. Dynamic CD8(+) T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell. (2023) 186:1127–1143 e1118. doi: 10.1016/j.cell.2023.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zander R, Schauder D, Xin G, Nguyen C, Wu X, Zajac A, et al. CD4(+) T cell help is required for the formation of a cytolytic CD8(+) T cell subset that protects against chronic infection and cancer. Immunity. (2019) 51:1028–1042 e1024. doi: 10.1016/j.immuni.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cui C, Wang J, Fagerberg E, Chen PM, Connolly KA, Damo M, et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell. (2021) 184:6101–6118 e6113. doi: 10.1016/j.cell.2021.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Im SJ, Obeng RC, Nasti TH, McManus D, Kamphorst AO, Gunisetty S, et al. Characteristics and anatomic location of PD-1(+)TCF1(+) stem-like CD8 T cells in chronic viral infection and cancer. Proc Natl Acad Sci USA. (2023) 120:e2221985120. doi: 10.1073/pnas.2221985120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. (2020) 577:549–55. doi: 10.1038/s41586-019-1922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. (2020) 577:556–60. doi: 10.1038/s41586-019-1906-8 [DOI] [PubMed] [Google Scholar]
- 71. Jansen CS, Prabhu RS, Pagadala MS, Chappa P, Goyal S, Zhou C, et al. Immune niches in brain metastases contain TCF1+ stem-like T cells, are associated with disease control and are modulated by preoperative SRS. Res Sq. (2023) 23:rs.3.rs-2722744. doi: 10.21203/rs.3.rs-2722744/v1 [DOI] [Google Scholar]
- 72. Carmona SJ, Siddiqui I, Bilous M, Held W, Gfeller D. Deciphering the transcriptomic landscape of tumor-infiltrating CD8 lymphocytes in B16 melanoma tumors with single-cell RNA-Seq. Oncoimmunology. (2020) 9:1737369. doi: 10.1080/2162402X.2020.1737369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. (2014) 26:638–52. doi: 10.1016/j.ccell.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meiser P, Knolle MA, Hirschberger A, de Almeida GP, Bayerl F, Lacher S, et al. A distinct stimulatory cDC1 subpopulation amplifies CD8(+) T cell responses in tumors for protective anti-cancer immunity. Cancer Cell. (2023) 41:1498–1515 e1410. doi: 10.1016/j.ccell.2023.06.008 [DOI] [PubMed] [Google Scholar]
- 75. Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. (2017) 355:1428–33. doi: 10.1126/science.aaf1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. (2017) 355:1423–7. doi: 10.1126/science.aaf0683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dangaj D, Bruand M, Grimm AJ, Ronet C, Barras D, Duttagupta PA, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell. (2019) 35:885–900 e810. doi: 10.1016/j.ccell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. House IG, Savas P, Lai J, Chen AXY, Oliver AJ, Teo ZL, et al. Macrophage-derived CXCL9 and CXCL10 are required for antitumor immune responses following immune checkpoint blockade. Clin Cancer Res. (2020) 26:487–504. doi: 10.1158/1078-0432.CCR-19-1868 [DOI] [PubMed] [Google Scholar]
- 79. Petty AJ, Li A, Wang X, Dai R, Heyman B, Hsu D, et al. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest. (2019) 129:5151–62. doi: 10.1172/JCI128644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rashidian M, LaFleur MW, Verschoor VL, Dongre A, Zhang Y, Nguyen TH, et al. Immuno-PET identifies the myeloid compartment as a key contributor to the outcome of the antitumor response under PD-1 blockade. Proc Natl Acad Sci USA. (2019) 116:16971–80. doi: 10.1073/pnas.1905005116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Qu Y, Wen J, Thomas G, Yang W, Prior W, He W, et al. Baseline frequency of inflammatory Cxcl9-expressing tumor-associated macrophages predicts response to avelumab treatment. Cell Rep. (2020) 32:107873. doi: 10.1016/j.celrep.2020.107873 [DOI] [PubMed] [Google Scholar]
- 82. Marcovecchio PM, Thomas G, Salek-Ardakani S. CXCL9-expressing tumor-associated macrophages: new players in the fight against cancer. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2020-002045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumor burden ratio associated with anti-PD-1 response. Nature. (2017) 545:60–5. doi: 10.1038/nature22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. (2018) 24:994–1004. doi: 10.1038/s41591-018-0057-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Clarke J, Panwar B, Madrigal A, Singh D, Gujar R, Wood O, et al. Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J Exp Med. (2019) 216:2128–49. doi: 10.1084/jem.20190249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang Y, Chen H, Mo H, Hu X, Gao R, Zhao Y, et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. (2021) 39:1578–1593 e1578. doi: 10.1016/j.ccell.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 87. Li K, Tandurella JA, Gai J, Zhu Q, Lim SJ, Thomas DL, 2nd, et al. Multi-omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti-PD-1 therapy. Cancer Cell. (2022) 40:1374–1391 e1377. doi: 10.1016/j.ccell.2022.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bassez A, Vos H, Van Dyck L, Floris G, Arijs I, Desmedt C, et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat Med. (2021) 27:820–32. doi: 10.1038/s41591-021-01323-8 [DOI] [PubMed] [Google Scholar]
- 89. Held W, Siddiqui I, Schaeuble K, Speiser DE. Intratumoral CD8(+) T cells with stem cell-like properties: Implications for cancer immunotherapy. Sci Transl Med. (2019) 11(515). doi: 10.1126/scitranslmed.aay6863 [DOI] [PubMed] [Google Scholar]
- 90. Liu B, Hu X, Feng K, Gao R, Xue Z, Zhang S, et al. Temporal single-cell tracing reveals clonal revival and expansion of precursor exhausted T cells during anti-PD-1 therapy in lung cancer. Nat Cancer. (2022) 3:108–21. doi: 10.1038/s43018-021-00292-8 [DOI] [PubMed] [Google Scholar]
- 91. Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med. (2015) 212:1125–37. doi: 10.1084/jem.20142237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tsui C, Kretschmer L, Rapelius S, Gabriel SS, Chisanga D, Knopper K, et al. MYB orchestrates T cell exhaustion and response to checkpoint inhibition. Nature. (2022) 609:354–60. doi: 10.1038/s41586-022-05105-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tichet M, Wullschleger S, Chryplewicz A, Fournier N, Marcone R, Kauzlaric A, et al. Bispecific PD1-IL2v and anti-PD-L1 break tumor immunity resistance by enhancing stem-like tumor-reactive CD8(+) T cells and reprogramming macrophages. Immunity. (2023) 56:162–179 e166. doi: 10.1016/j.immuni.2022.12.006 [DOI] [PubMed] [Google Scholar]
- 94. Philipp N, Kazerani M, Nicholls A, Vick B, Wulf J, Straub T, et al. T-cell exhaustion induced by continuous bispecific molecule exposure is ameliorated by treatment-free intervals. Blood. (2022) 140:1104–18. doi: 10.1182/blood.2022015956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lelliott EJ, Kong IY, Zethoven M, Ramsbottom KM, Martelotto LG, Meyran D, et al. CDK4/6 inhibition promotes antitumor immunity through the induction of T-cell memory. Cancer Discovery. (2021) 11:2582–601. doi: 10.1158/2159-8290.CD-20-1554 [DOI] [PubMed] [Google Scholar]
- 96. Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity. (2016) 44:609–21. doi: 10.1016/j.immuni.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 97. Verma V, Jafarzadeh N, Boi S, Kundu S, Jiang Z, Fan Y, et al. MEK inhibition reprograms CD8(+) T lymphocytes into memory stem cells with potent antitumor effects. Nat Immunol. (2021) 22:53–66. doi: 10.1038/s41590-020-00818-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. LaFleur MW, Nguyen TH, Coxe MA, Miller BC, Yates KB, Gillis JE, et al. PTPN2 regulates the generation of exhausted CD8(+) T cell subpopulations and restrains tumor immunity. Nat Immunol. (2019) 20:1335–47. doi: 10.1038/s41590-019-0480-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pelly VS, Moeini A, Roelofsen LM, Bonavita E, Bell CR, Hutton C, et al. Anti-inflammatory drugs remodel the tumor immune environment to enhance immune checkpoint blockade efficacy. Cancer Discovery. (2021) 11:2602–19. doi: 10.1158/2159-8290.CD-20-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Liu C, Somasundaram A, Manne S, Gocher AM, Szymczak-Workman AL, Vignali KM, et al. Neuropilin-1 is a T cell memory checkpoint limiting long-term antitumor immunity. Nat Immunol. (2020) 21:1010–21. doi: 10.1038/s41590-020-0733-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Francis DM, Manspeaker MP, Schudel A, Sestito LF, O’Melia MJ, Kissick HT, et al. Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy. Sci Transl Med. (2020) 12(563). doi: 10.1126/scitranslmed.aay3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shen Y, Connolly E, Aiello M, Zhou C, Chappa P, Song H, et al. Radiation and anti-PD-L1 synergize by stimulating a stem-like T cell population in the tumor-draining lymph node. Res Sq. (2024) 6:rs.3.rs-3921977. doi: 10.1158/1538-7445.AM2024-LB084 [DOI] [Google Scholar]
- 103. Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. (2013) 121:573–84. doi: 10.1182/blood-2012-05-431718 [DOI] [PubMed] [Google Scholar]
- 104. Sabatino M, Hu J, Sommariva M, Gautam S, Fellowes V, Hocker JD, et al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell Malignancies. Blood. (2016) 128:519–28. doi: 10.1182/blood-2015-11-683847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zanon V, Pilipow K, Scamardella E, De Paoli F, De Simone G, Price DA, et al. Curtailed T-cell activation curbs effector differentiation and generates CD8(+) T cells with a naturally-occurring memory stem cell phenotype. Eur J Immunol. (2017) 47:1468–76. doi: 10.1002/eji.201646732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lee J, Lee K, Bae H, Lee K, Lee S, Ma J, et al. IL-15 promotes self-renewal of progenitor exhausted CD8 T cells during persistent antigenic stimulation. Front Immunol. (2023) 14:1117092. doi: 10.3389/fimmu.2023.1117092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Romine KA, MacPherson K, Cho HJ, Kosaka Y, Flynn PA, Byrd KH, et al. BET inhibitors rescue anti-PD1 resistance by enhancing TCF7 accessibility in leukemia-derived terminally exhausted CD8(+) T cells. Leukemia. (2023) 37:580–92. doi: 10.1038/s41375-023-01808-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kondo T, Morita R, Okuzono Y, Nakatsukasa H, Sekiya T, Chikuma S, et al. Notch-mediated conversion of activated T cells into stem cell memory-like T cells for adoptive immunotherapy. Nat Commun. (2017) 8:15338. doi: 10.1038/ncomms15338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ando M, Kondo T, Tomisato W, Ito M, Shichino S, Srirat T, et al. Rejuvenating effector/exhausted CAR T cells to stem cell memory-like CAR T cells by resting them in the presence of CXCL12 and the NOTCH ligand. Cancer Res Commun. (2021) 1:41–55. doi: 10.1158/2767-9764.CRC-21-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kagoya Y, Nakatsugawa M, Yamashita Y, Ochi T, Guo T, Anczurowski M, et al. BET bromodomain inhibition enhances T cell persistence and function in adoptive immunotherapy models. J Clin Invest. (2016) 126:3479–94. doi: 10.1172/JCI86437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mousset CM, Hobo W, Ji Y, Fredrix H, De Giorgi V, Allison RD, et al. Ex vivo AKT-inhibition facilitates generation of polyfunctional stem cell memory-like CD8(+) T cells for adoptive immunotherapy. Oncoimmunology. (2018) 7:e1488565. doi: 10.1080/2162402X.2018.1488565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. (2005) 115:1616–26. doi: 10.1172/JCI24480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. (2009) 15:808–13. doi: 10.1038/nm.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. (2011) 17:1290–7. doi: 10.1038/nm.2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chapuis AG, Ragnarsson GB, Nguyen HN, Chaney CN, Pufnock JS, Schmitt TM, et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. (2013) 5:174ra127. doi: 10.1126/scitranslmed.3004916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. (2014) 123:3750–9. doi: 10.1182/blood-2014-01-552174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. (2018) 24:563–71. doi: 10.1038/s41591-018-0010-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pilipow K, Scamardella E, Puccio S, Gautam S, De Paoli F, Mazza EM, et al. Antioxidant metabolism regulates CD8+ T memory stem cell formation and antitumor immunity. JCI Insight. (2018) 3(18). doi: 10.1172/jci.insight.122299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. (2019) 380:45–56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 121. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicenter seamless design study. Lancet. (2020) 396:839–52. doi: 10.1016/S0140-6736(20)31366-0 [DOI] [PubMed] [Google Scholar]
- 122. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-Line therapy for large B-Cell lymphoma. New Engl J Med. (2022) 386:640–54. doi: 10.1056/NEJMoa2116133 [DOI] [PubMed] [Google Scholar]
- 123. Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomized, phase 3 trial. Lancet. (2022) 399:2294–308. doi: 10.1016/S0140-6736(22)00662-6 [DOI] [PubMed] [Google Scholar]
- 124. Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicenter, phase 2 trial. Lancet Oncol. (2022) 23:91–103. doi: 10.1016/S1470-2045(21)00591-X [DOI] [PubMed] [Google Scholar]
- 125. Fowler NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. (2022) 28:325–32. doi: 10.1038/s41591-021-01622-0 [DOI] [PubMed] [Google Scholar]
- 126. Deng Q, Han G, Puebla-Osorio N, Ma MCJ, Strati P, Chasen B, et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med. (2020) 26:1878–87. doi: 10.1038/s41591-020-1061-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. (2020) 4:4898–911. doi: 10.1182/bloodadvances.2020002394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Monfrini C, Stella F, Aragona V, Magni M, Ljevar S, Vella C, et al. Phenotypic composition of commercial anti-CD19 CAR T cells affects in vivo expansion and disease response in patients with large B-cell lymphoma. Clin Cancer Res. (2022) 28:3378–86. doi: 10.1158/1078-0432.CCR-22-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Denlinger N, Song NJ, Zhang X, Jeon H, Peterson C, Wang Y, et al. Postinfusion PD-1+ CD8+ CAR T cells identify patients responsive to CD19 CAR T-cell therapy in non-Hodgkin lymphoma. Blood Adv. (2024) 8:3140–53. doi: 10.1182/bloodadvances.2023012073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lynn RC, Weber EW, Sotillo E, Gennert D, Xu P, Good Z, et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. (2019) 576:293–300. doi: 10.1038/s41586-019-1805-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chan JD, Scheffler CM, Munoz I, Sek K, Lee JN, Huang YK, et al. FOXO1 enhances CAR T cell stemness, metabolic fitness and efficacy. Nature. (2024) 629:201–10. doi: 10.1038/s41586-024-07242-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jung IY, Narayan V, McDonald S, Rech AJ, Bartoszek R, Hong G, et al. BLIMP1 and NR4A3 transcription factors reciprocally regulate antitumor CAR T cell stemness and exhaustion. Sci Transl Med. (2022) 14:eabn7336. doi: 10.1126/scitranslmed.abn7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Jiang P, Zhang Z, Hu Y, Liang Z, Han Y, Li X, et al. Single-cell ATAC-seq maps the comprehensive and dynamic chromatin accessibility landscape of CAR-T cell dysfunction. Leukemia. (2022) 36:2656–68. doi: 10.1038/s41375-022-01676-0 [DOI] [PubMed] [Google Scholar]
- 134. Chen J, Lopez-Moyado IF, Seo H, Lio CJ, Hempleman LJ, Sekiya T, et al. NR4A transcription factors limit CAR T cell function in solid tumors. Nature. (2019) 567:530–4. doi: 10.1038/s41586-019-0985-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhu Z, Lou G, Teng XL, Wang H, Luo Y, Shi W, et al. FOXP1 and KLF2 reciprocally regulate checkpoints of stem-like to effector transition in CAR T cells. Nat Immunol. (2024) 25:117–28. doi: 10.1038/s41590-023-01685-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Scholler N, Perbost R, Locke FL, Jain MD, Turcan S, Danan C, et al. Tumor immune contexture is a determinant of anti-CD19 CAR T cell efficacy in large B cell lymphoma. Nat Med. (2022) 28:1872–82. doi: 10.1038/s41591-022-01916-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Reinhard K, Rengstl B, Oehm P, Michel K, Billmeier A, Hayduk N, et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. (2020) 367:446–53. doi: 10.1126/science.aay5967 [DOI] [PubMed] [Google Scholar]
- 138. Mizukoshi E, Nakagawa H, Tamai T, Kitahara M, Fushimi K, Nio K, et al. long-term surviving cancer patients harbor self-renewing tumor-specific CD8(+) T cells. Nat Commun. (2022) 13:3123. doi: 10.1038/s41467-022-30861-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. (2017) 547:222–6. doi: 10.1038/nature23003 [DOI] [PubMed] [Google Scholar]
- 140. Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. (2017) 547:217–21. doi: 10.1038/nature22991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. (2015) 520:692–6. doi: 10.1038/nature14426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumor mutations by combining mass spectrometry and exome sequencing. Nature. (2014) 515:572–6. doi: 10.1038/nature14001 [DOI] [PubMed] [Google Scholar]
- 143. Li B, Jing P, Zheng G, Pi C, Zhang L, Yin Z, et al. Neo-intline: integrated pipeline enables neoantigen design through the in-silico presentation of T-cell epitope. Signal Transduct Target Ther. (2023) 8:397. doi: 10.1038/s41392-023-01644-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanovic S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. (2019) 565:240–5. doi: 10.1038/s41586-018-0810-y [DOI] [PubMed] [Google Scholar]
- 145. Yu YJ, Shan N, Li LY, Zhu YS, Lin LM, Mao CC, et al. Preliminary clinical study of personalized neoantigen vaccine therapy for microsatellite stability (MSS)-advanced colorectal cancer. Cancer Immunol Immunother. (2023) 72:2045–56. doi: 10.1007/s00262-023-03386-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Baharom F, Ramirez-Valdez RA, Tobin KKS, Yamane H, Dutertre CA, Khalilnezhad A, et al. Intravenous nanoparticle vaccination generates stem-like TCF1(+) neoantigen-specific CD8(+) T cells. Nat Immunol. (2021) 22:41–52. doi: 10.1038/s41590-020-00810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. D’Alise AM, Brasu N, De Intinis C, Leoni G, Russo V, Langone F, et al. Adenoviral-based vaccine promotes neoantigen-specific CD8(+) T cell stemness and tumor rejection. Sci Transl Med. (2022) 14:eabo7604. doi: 10.1126/scitranslmed.abo7604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Shimizu K, Ueda S, Kawamura M, Aoshima H, Satoh M, Nakabayashi J, et al. Combination of cancer vaccine with CD122-biased IL-2/anti-IL-2 Ab complex shapes the stem-like effector NK and CD8(+) T cells against tumor. J Immunother Cancer. (2023) 11(7). doi: 10.1136/jitc-2022-006409 [DOI] [PMC free article] [PubMed] [Google Scholar]