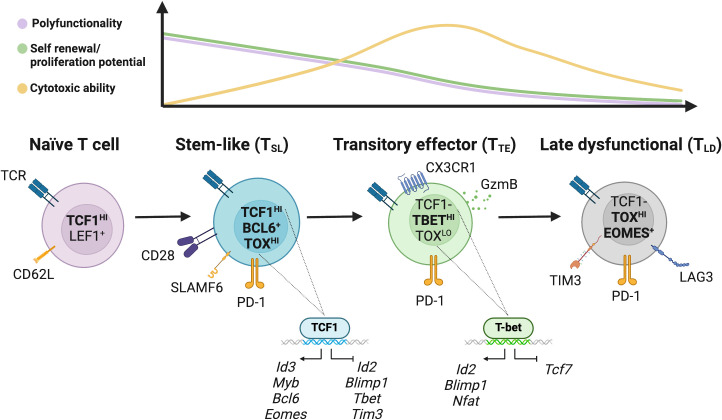
Figure 2.
Model of the characteristics and differentiation of CD8+ T cell states in cancer. The transcription factors TCF1 and LEF1, as well as the adhesion and lymphocyte homing molecules CD62L, are highly expressed in naïve T cells. Downstream, the population of stem-like CD8+ T (TSL) cells with strong polyfunctionality and self-renewal ability is defined by TCF1. These cells have a strong proliferation capacity, are primarily quiescent in vivo, and are able to support the CD8+ T cell response. By suppressing the expression of effector-associated genes like Id2, Blimp-1, Tbet and Tbx21 and stimulating memory-associated genes like Eomes, Myb, Bcl-6, TCF1 facilitates the generation, maintenance, and functionality of these cells. Phenotypically, TSL cells display CD28 and ICOS costimulatory markers, low or moderate amounts of PD-1, SLAMF6, and CXCR5. TSL cells expand upon ICB and both maintain the TSL reservoir and differentiate into further subpopulations. Differentiation of TSL give rise to downstream transitory effector CD8+ T cells (TTE) that express high PD-1 receptor, proliferate rapidly in steady state down regulating TCF1 expression, and upregulate T-BET. T-BET inhibits TOX-mediated development of late dysfunctional T cells (TLD) phenotype in TTE cells. Transitory cells exhibit the expression of CX3 chemokine receptor 1 (CX3CR1) and PD-1. TTE proliferate to help target and eliminate tumor cells. After chronic antigen stimulation, these TTE cells develop into TLD cells which are characterized by high expression of checkpoint receptors (PD-1, TIM3, LAG3, CTLA-4, TIGIT, and CD101), poor polyfunctionality, low proliferation capacity, but retain some cytotoxic potential.