Abstract
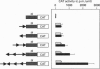
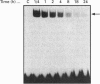
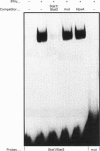
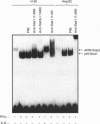
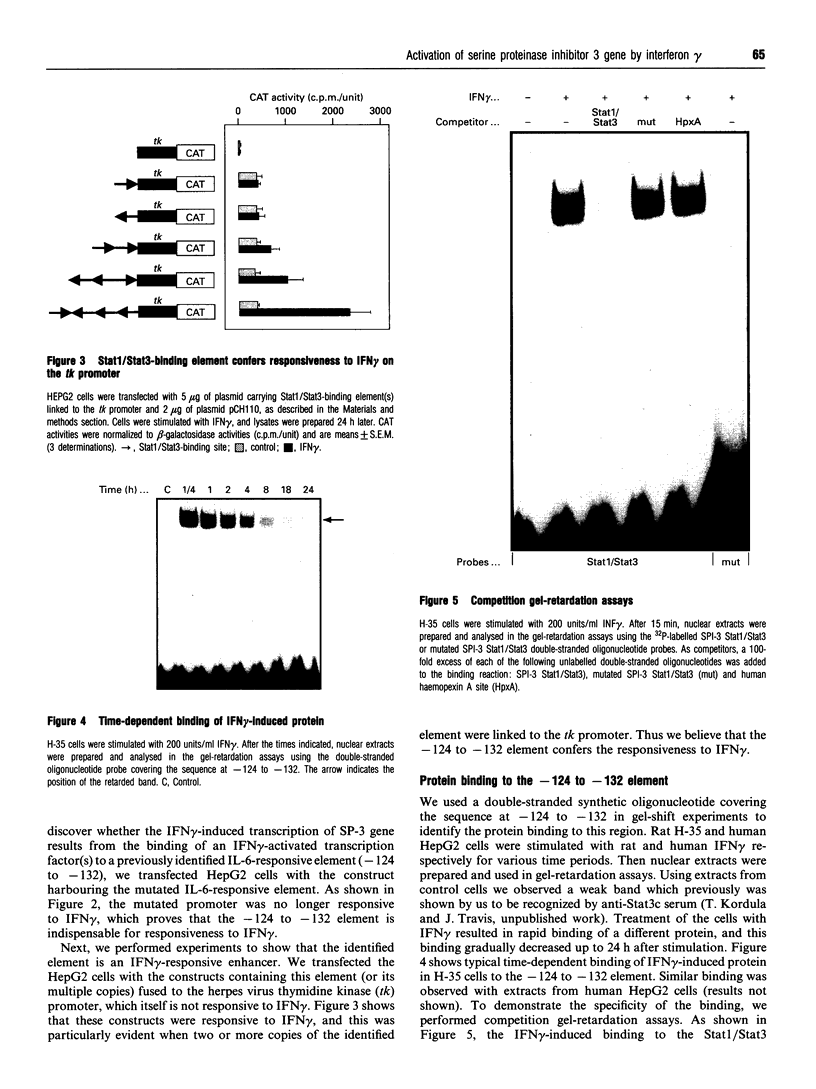
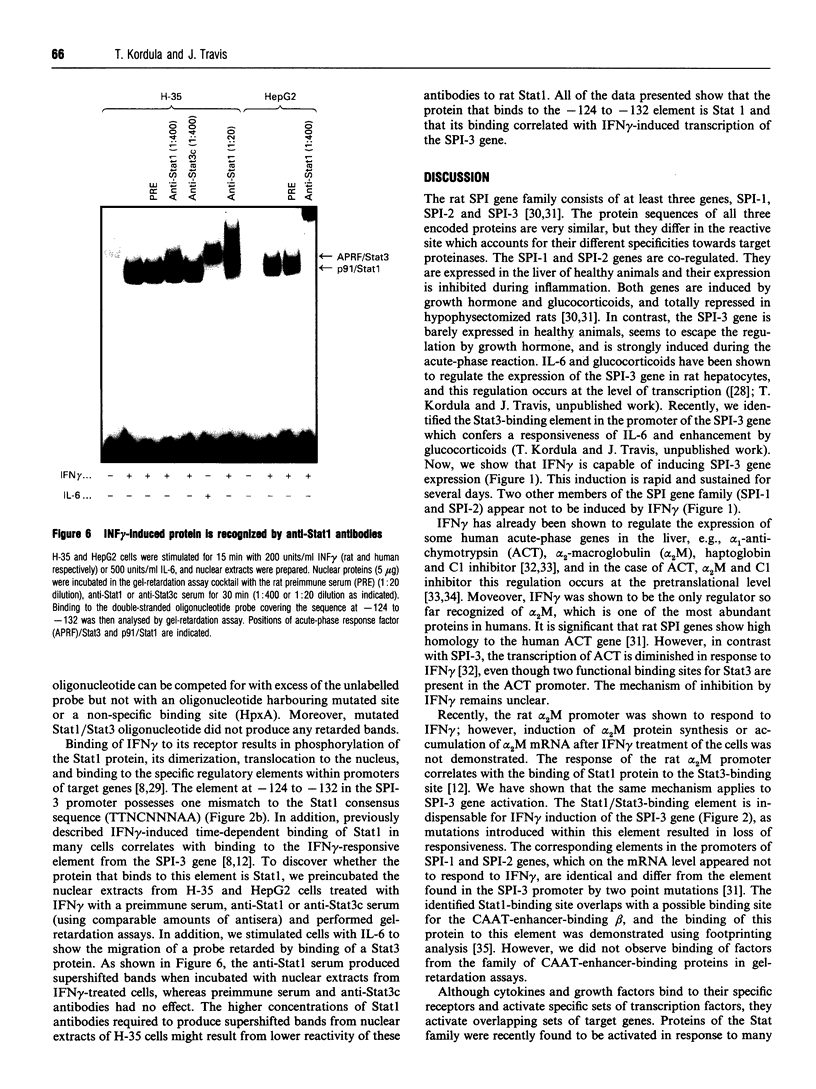
Transcription of rat serine proteinase inhibitor 3 (SPI-3) gene is rapidly induced in the liver in response to inflammation. Treatment of rat hepatoma H-35 cells with interferon gamma (INF gamma) results in the immediate induction of this gene, with its 147 bp-long promoter being sufficient for activation. Within this promoter we have identified an IFN gamma-responsive element which maps to the signal transducer and activator of transcription (Stat)3-binding site. Mutation of this element causes a loss of responsiveness to IFN gamma, whereas fusion to a heterologous promoter confers a positive response on IFN gamma. The latter apparently induces the binding of a protein, identified as Stat1, to the described element, which gradually decreases within 24 h. Thus the induction of the SPI-3 gene by IFN gamma correlates with the binding of Stat1 to a specific element which, in turn, binds Stat3 in response to interleukin 6.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdollahi A., Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Interferon regulatory factor 1 is a myeloid differentiation primary response gene induced by interleukin 6 and leukemia inhibitory factor: role in growth inhibition. Cell Growth Differ. 1991 Aug;2(8):401–407. [PubMed] [Google Scholar]
- Baumann H., Gauldie J. The acute phase response. Immunol Today. 1994 Feb;15(2):74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Baumann H., Prowse K. R., Marinković S., Won K. A., Jahreis G. P. Stimulation of hepatic acute phase response by cytokines and glucocorticoids. Ann N Y Acad Sci. 1989;557:280-95, discussion 295-6. doi: 10.1111/j.1749-6632.1989.tb24021.x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Delegeane A. M., Ferland L. H., Mellon P. L. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Mol Cell Biol. 1987 Nov;7(11):3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar M. A., Schreiber R. D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harroch S., Revel M., Chebath J. Induction by interleukin-6 of interferon regulatory factor 1 (IRF-1) gene expression through the palindromic interferon response element pIRE and cell type-dependent control of IRF-1 binding to DNA. EMBO J. 1994 Apr 15;13(8):1942–1949. doi: 10.1002/j.1460-2075.1994.tb06463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. E., Hastie N. D. Accelerated evolution in the reactive centre regions of serine protease inhibitors. Nature. 1987 Mar 5;326(6108):96–99. doi: 10.1038/326096a0. [DOI] [PubMed] [Google Scholar]
- Kordula T., Bugno M., Lason W., Przewlocki R., Koj A. Rat contrapsins are the type II acute phase proteins: regulation by interleukin 6 on the mRNA level. Biochem Biophys Res Commun. 1994 May 30;201(1):222–227. doi: 10.1006/bbrc.1994.1692. [DOI] [PubMed] [Google Scholar]
- Kordula T., Rose-John S., Heinrich P. C., Koj A. Interferon-gamma regulates alpha 2-macroglobulin and alpha 1-antichymotrypsin expression on the pretranslational level in HepG2 cells. Folia Histochem Cytobiol. 1992;30(4):147–149. [PubMed] [Google Scholar]
- Lütticken C., Wegenka U. M., Yuan J., Buschmann J., Schindler C., Ziemiecki A., Harpur A. G., Wilks A. F., Yasukawa K., Taga T. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994 Jan 7;263(5143):89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Pagès G., Rouayrenc J. F., Rossi V., Le Cam G., Mariller M., Szpirer J., Szpirer C., Levan G., Le Cam A. Primary structure and assignment to chromosome 6 of three related rat genes encoding liver serine protease inhibitors. Gene. 1990 Oct 15;94(2):273–282. doi: 10.1016/0378-1119(90)90398-b. [DOI] [PubMed] [Google Scholar]
- Poli V., Mancini F. P., Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990 Nov 2;63(3):643–653. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Dietrich A., Marks F. Molecular cloning of mouse protein kinase C (PKC) cDNA from Swiss 3T3 fibroblasts. Gene. 1988 Dec 30;74(2):465–471. doi: 10.1016/0378-1119(88)90179-5. [DOI] [PubMed] [Google Scholar]
- Rossi V., Rouayrenc J. F., Paquereau L., Vilarem M. J., Le Cam A. Analysis of proteins binding to the proximal promoter region of two rat serine protease inhibitor genes. Nucleic Acids Res. 1992 Mar 11;20(5):1061–1068. doi: 10.1093/nar/20.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Sadowski H. B., Shuai K., Darnell J. E., Jr, Gilman M. Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Van Dyke M. W., Gregor P. D., Roeder R. G. Multiple forms of the human gene-specific transcription factor USF. I. Complete purification and identification of USF from HeLa cell nuclei. J Biol Chem. 1988 Aug 25;263(24):11985–11993. [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Shuai K., Ziemiecki A., Wilks A. F., Harpur A. G., Sadowski H. B., Gilman M. Z., Darnell J. E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993 Dec 9;366(6455):580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Stahl N., Boulton T. G., Farruggella T., Ip N. Y., Davis S., Witthuhn B. A., Quelle F. W., Silvennoinen O., Barbieri G., Pellegrini S. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994 Jan 7;263(5143):92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yamamoto K., Sinohara H. Molecular cloning and sequence analysis of full-length cDNA coding for mouse contrapsin. J Biochem. 1990 Sep;108(3):344–346. doi: 10.1093/oxfordjournals.jbchem.a123204. [DOI] [PubMed] [Google Scholar]
- Wegenka U. M., Buschmann J., Lütticken C., Heinrich P. C., Horn F. Acute-phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol. 1993 Jan;13(1):276–288. doi: 10.1128/mcb.13.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Wegenka U. M., Lütticken C., Buschmann J., Decker T., Schindler C., Heinrich P. C., Horn F. The signalling pathways of interleukin-6 and gamma interferon converge by the activation of different transcription factors which bind to common responsive DNA elements. Mol Cell Biol. 1994 Mar;14(3):1657–1668. doi: 10.1128/mcb.14.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994 Apr 1;264(5155):95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- Zuraw B. L., Lotz M. Regulation of the hepatic synthesis of C1 inhibitor by the hepatocyte stimulating factors interleukin 6 and interferon gamma. J Biol Chem. 1990 Jul 25;265(21):12664–12670. [PubMed] [Google Scholar]