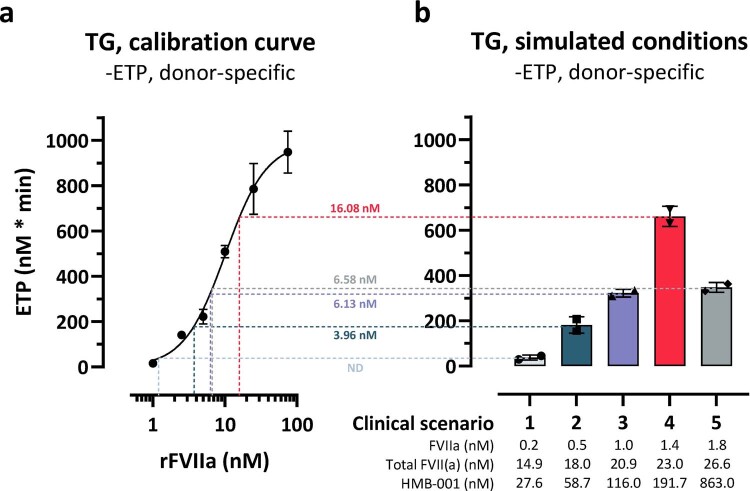
Extended Data Fig. 7. Representative data from a single donor showing enhanced FVIIa activity by HMB-001 at predicted plasma levels of FVIIa, total FVII(a) and HMB-001 following five different dosing regimens.
rFVIIa-equivalent activity was measured in PRP from a healthy control (n = 3) supplemented with d-RGDW to obtain GT-like platelets and a blocking anti-TF antibody to ensure TF-independence. PRP was supplemented with rFVIIa (0–75 nM), platelets were stimulated with CRP-XL and PAR-1 AP, and thrombin generation was measured for 240 min. a) For each donor, Endogenous thrombin potential (ETP) was determined in duplicate as a function of rFVIIa concentration. Data represent mean ± s.d. b) ETP was determined in duplicate at 5 predicted clinical scenarios of FVIIa, total FVII(a) and HMB-001 in healthy controls (n = 3). The background plasma concentrations of FVIIa and total FVII(a) were assumed to be 67 pM and 10 nM, respectively. Predicted accumulated levels of total FVII(a) and FVIIa for 5 predicted clinical scenarios (Table S8) were reconstituted by adding FVII-S195A and FVIIa after accounting for the background plasma concentration of total FVII(a) and FVIIa respectively. HMB-001 was added as indicated. Data represent mean ± s.d. For each clinical scenario, the corresponding rFVIIa-equivalent activity (nM), as extrapolated from the X-axis of the calibration curve, is indicated next to the horizontal dotted line for the single donor. rFVIIa-equivalent activities for three donors are shown in Fig. 7G.