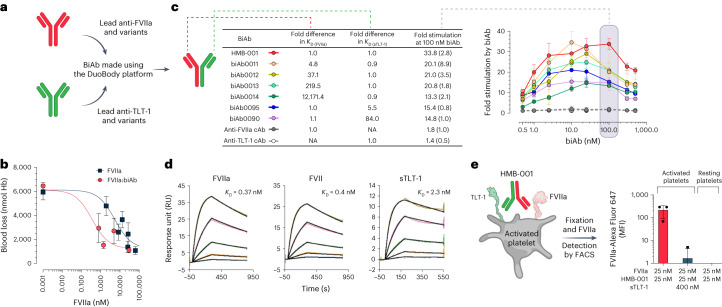
Fig. 2. Optimal affinity for FVIIa and TLT-1 binding by HMB-001 leads to efficient TLT-1-dependent FVIIa localization on the activated platelet.
a, BiAbs were made using the DuoBody platform. b, The ability of the biAb to potentiate FVIIa activity in vivo was evaluated in transgenic HA mice expressing human TLT-1 and using the TVT injury model. Anesthetized mice were placed on a heating pad set to maintain animal body temperature and with the tail submerged in saline (37 °C). FVIIa alone (n = 3–10, dark squares) or coformulated with an equimolar concentration of the biAb (n = 6, red circles) was administered intravenously into the right lateral tail vein 5 min before the injury. Total blood loss was determined by quantifying the amount of hemoglobin (Hb) in the saline and is expressed as nmol Hb. FVIIa concentrations were measured at the end of the bleeding window. Data are expressed as mean blood loss ± s.d. c, BiAbs with different affinities, as measured using surface plasmon resonance (SPR) technology (n = 2), toward FVIIa and sTLT-1 were generated. FX (150 nM) was activated with FVIIa (2.5 nM) in the presence of lipidated TLT-1 and biAbs at the indicated concentrations for 20 min (n = 3). Reactions were quenched, and FXa formation was assessed with a chromogenic substrate. At the anticipated clinically relevant biAb plasma concentration of 100 nM, shown in the shaded gray region, data are expressed as mean fold-stimulation in FXa generation compared to the absence of the biAb. Error bars indicate s.d. NA, not applicable. d, Binding of HMB-001 to FVIIa, zymogen FVII and sTLT-1 was assessed with SPR technology at 25 °C and pH 7.4 (n = 2). Kinetic data were fitted to a Langmuir 1:1 binding model to obtain KD values. e, Whole blood from healthy donors (n = 3) was incubated with FVIIa, HMB-001 or sTLT-1, as indicated, in the presence of an Alexa Fluor 647-labeled FVIIa-specific VHH with or without a cocktail of 25 mM PAR-1 AP and 1 mg ml−1 CRP-XL for 10 min. FVIIa binding to platelets was assessed with FACS. Data are expressed as mean MFI ± s.d.