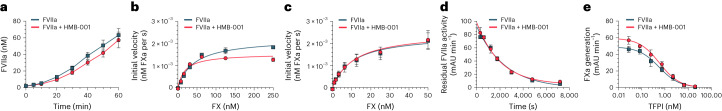
Fig. 3. HMB-001 does not interfere with the normal functioning of FVIIa.
a, FVII autoactivation. FVII (145 nM) was activated with FVIIa (2 nM) in the presence of lipidated TF (2 nM) and 0 or 500 nM HMB-001 for 0–60 min (n = 3). FVIIa activity was assessed with a chromogenic substrate in the presence of 200 nM lipidated TF. Data are expressed as mean ± s.d. b, TF-independent FX activation. Human plasma-derived FX (0–250 nM) was activated with FVIIa (20 nM) in the presence of 0 or 500 nM HMB-001 for 20 min (n = 3). FXa was assessed with a chromogenic substrate. FXa generation rates (nM FXa per s) were plotted as a function of FX concentration. Data are expressed as mean ± s.d. c, TF-dependent FX activation. Human plasma-derived FX (0–50 nM) was activated with FVIIa (100 pM) in the presence of 0 or 50 nM HMB-001 and 2 pM lipidated TF for 20 min (n = 3). FXa was assessed with a chromogenic substrate. FXa generation rates (nM FXa per s) were plotted as a function of FX concentration. Data are expressed as mean ± s.d. d, FVIIa inactivation by AT. FVIIa (200 nM) was preincubated with 12 mM low-molecular-weight heparin and 0 or 500 nM HMB-001 for 10 min, followed by incubation with AT for 0–2 h (n = 3). Residual FVIIa activity (mAU min−1) was assessed with a chromogenic substrate and plotted as a function of time. Data are expressed as mean ± s.d. e, FVIIa inactivation by TFPI. FVIIa (100 pM) was preincubated with 2 pM TF, 0–20 nM TFPI and 0 or 500 nM HMB-001 for 10 min, followed by incubation with 50 nM FX for 30 min (n = 3). FXa activity was assessed with a chromogenic substrate, and residual FXa activity (mAU min−1) was plotted as a function of TFPI concentration. Data are expressed as mean ± s.d.