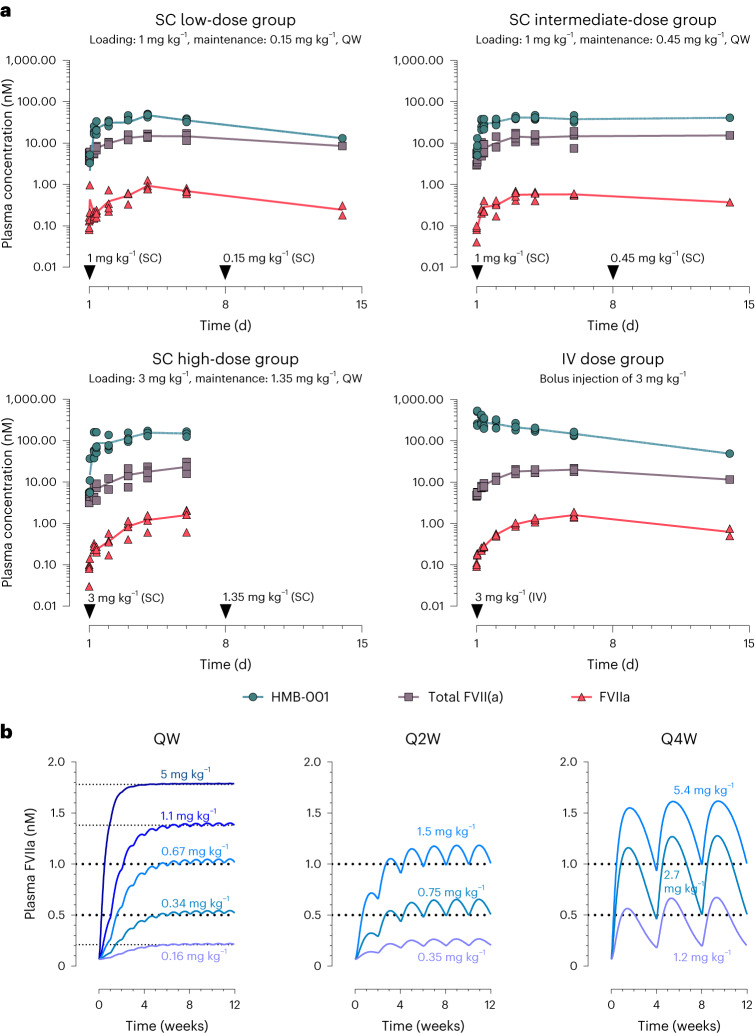
Fig. 6. HMB-001 leads to time- and dose-dependent accumulation of endogenous FVIIa and total FVII(a).
a, PK results of HMB-001 and time course of FVIIa and total FVII(a) accumulation in cynomolgus monkeys following subcutaneous (SC) and intravenous (IV) administration of HMB-001. Three groups (n = 4) were administered HMB-001 subcutaneously once weekly (QW), with clinically relevant loading and maintenance doses of 1 and 0.15, 1 and 0.45, and 3 and 1.35 mg kg−1, respectively. A fourth group (n = 4) was administered an intravenous bolus injection of 3 mg kg−1 HMB-001. Measured plasma concentrations of FVIIa (red triangles), total FVII(a) (gray squares) and HMB-001 (green circles) are shown for individual animals. For clarity purposes, only data points from ADA-negative plasma samples are shown. Solid lines connect the calculated means for each time point. Cynomolgus monkey FVIIa and total FVII(a) were measured using modified human FVIIa clot activity and human FVII ELISA kits (Stago) and, hence, are referred to as human-equivalent levels (Methods). b, Simulation of multiple-dose subcutaneous administration of HMB-001 in humans using a PK/PD model scaled to the human setting. For once-weekly simulations, five different clinical scenarios were simulated to identify the HMB-001 once-weekly dose to reach target accumulated endogenous FVIIa levels of 0.21, 0.52, 1, 1.38 and 1.78 nM (shown by horizontal dotted lines). Corresponding levels of total FVII(a) and HMB-001 are summarized in Supplementary Table 8. Every-2-week (Q2W) and every-4-week (Q4W) simulations were undertaken to show that endogenous FVIIa can be accumulated to target levels of 0.5 and 1 nM (horizontal dotted lines) with less frequent dosing regimens. HMB-001 doses predicted to be needed to reach each target FVIIa level are shown in blue and purple.