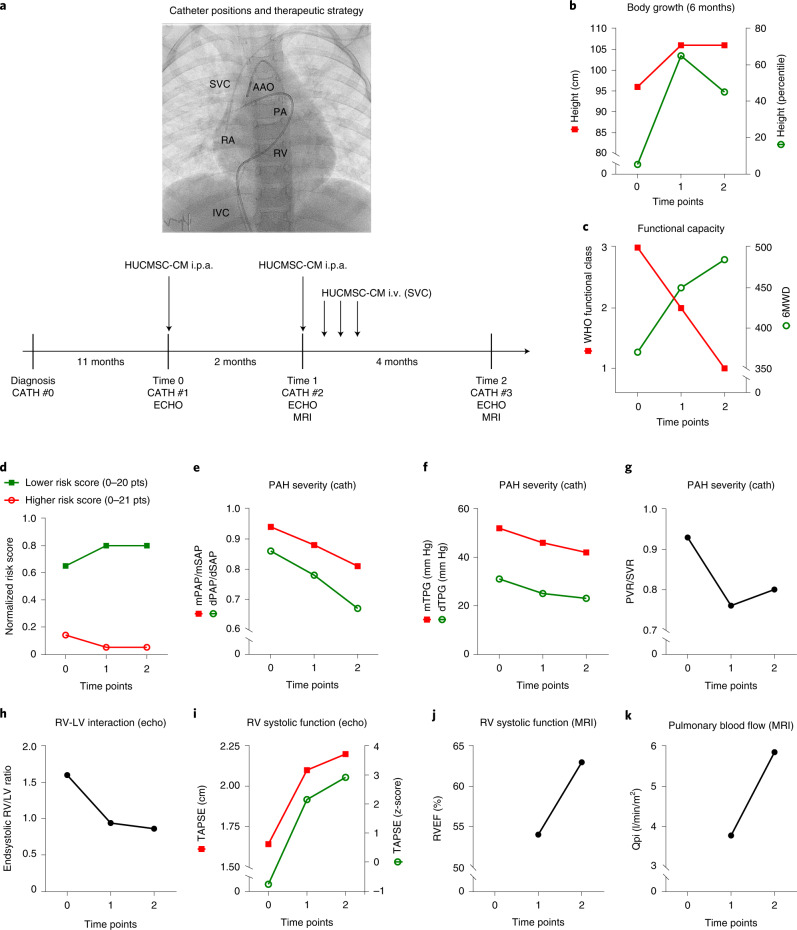
Fig. 1. Treatment with HUCMSC-CM results in improvement of growth, functional capacity, risk scores and multiple hemodynamic variables.
a, Four catheterizations (CATH #0, #1, #2 and #3) were performed spaced 11 months, 2 months and 4 months apart (shown in the timeline as vertical bars). The PAH medication was not changed within the 6 months before CATH #1 (Time 0, at our institution), when, for the first time, HUCMSC-CM was administered in the PAs. The PAH medication was also not changed thereafter. During CATH #1 (Time 0) and CATH #2 (Time 1), after full invasive hemodynamic assessment, 200 ml of HUCMSC-CM was infused in the PAs over 1 hour (100 ml into the right PA over 30 minutes; 100 ml into the left PA over 30 minutes). In the week after CATH #2 (Time 1), 200 ml of HUCMSC-CM was infused via a central venous line on days 1, 2 and 3 after CATH #2. Improvements in body growth (b), functional capacity (c), EPPVDN risk scores (d) and key morphological and hemodynamic data (e–k) as assessed by cardiac catheterization, echocardiography and cardiac magnetic resonance imaging were generated at the time points specified above (Times 0–2). See Methods for methodological details. cath, right and left heart catheterization; dTPG, diastolic transpulmonary pressure gradient; echo, transthoracic echocardiography; MRI, cardiac magnetic resonance imaging; mTPG, mean transpulmonary pressure gradient; Qpi, pulmonary blood flow index; SVR, systemic vascular resistance.