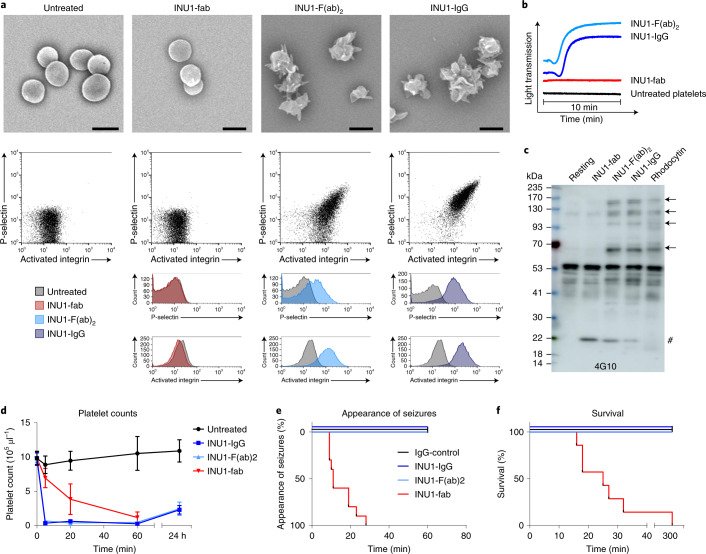
Fig. 1. CLEC-2 dimerization, but not binding of INU1-fab triggers platelet activation.
a, SEM (upper panels, scale 2 µm) and flow cytometry (lower panels) reveal platelet activation on binding of a divalent agent (INU1-IgG or INU1-F(ab)2), but not in response to INU1-fab (all antibody derivatives were added at 10 µg ml−1 final concentration [f.c.]) in vitro. Representative images of n = 4 per group. Gating strategy is indicated in Extended Data Fig. 1.b, INU1-IgG or INU1-F(ab)2, but not the monovalent INU1-fab trigger platelet aggregation in vitro (all antibody derivatives were added at 10 µg ml−1 f.c.; shown are representative traces for n = 10 each). c, Only multivalent CLEC-2 ligands, such as the snake venom rhodocytin or bivalent INU1-IgG, but not INU1-fab trigger CLEC-2 signaling in vitro as assessed by a tyrosine phosphorylation western blot (4G10). Newly phosphorylated proteins are indicated by arrows, the hash symbol indicates the band originating from the detection of INU1-fab by the secondary antibody. The depicted blot is representative of three independent experiments. d–f, Administration of INU1-F(ab)2 (0.5 µg g−1) or INU1-IgG (0.75 µg g−1) results in immediate platelet consumption, while INU1-fab (0.5 µg g−1) results in slowly progressing platelet consumption in vivo; depicted are mean ± s.d. (d). In contrast, in vivo only INU1-fab, but not INU1-F(ab)2 or INU1-IgG result in neurological impairment, like seizures (e) and is associated with lethality; untreated n = 5 (for all time points, except for 0 min where n = 7), INU1-IgG; n = 6–10, INU1-F(ab)2; n = 5–10, INU1-fab; n = 6–10, biologically independent (f). n = 5–10 mice per group; see source data files for exact numbers.