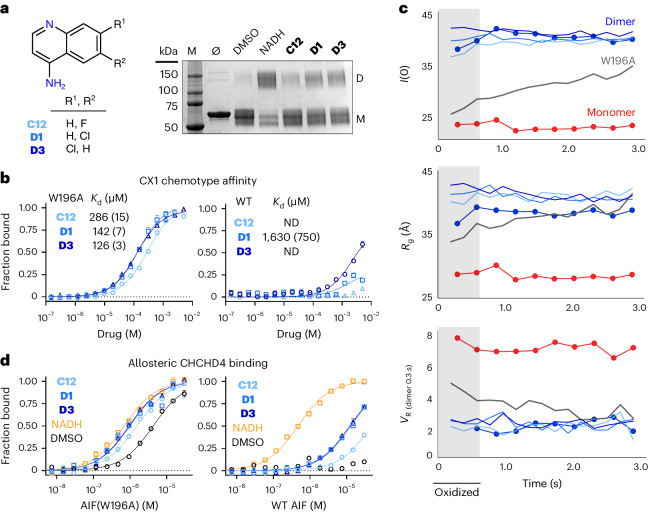
Fig. 4. The top-ranked SAXS chemotype selectively binds dimeric AIF and stimulates CHCHD4 binding.
a, Left, scheme for the CX1 4-aminoquinoline scaffold. Right, CX1 fragments induce weak AIF dimerization captured by bis(sulfosuccinimidyl)suberate (BS3) amine cross-linking; M, molecular weight marker lane. The displayed gel is representative of three independent experiments. b, CX1 fragments selectively bind dimer-permissive Atto488-labeled AIF(W196A) (left) over the wild-type (WT) AIF monomer (right) by MST. Standard errors of fitting are included in parentheses. Each curve represents the average of three thermophoresis scans of a representative binding titration; error bars represent standard deviations ND, affinity not determined. c, TR-SAXS analysis of AIF(W196A)–CX1 complexes. SAXS parameters for AIF(W196A) saturated with C12 (light blue), D1 (medium blue), D3 (dark blue) or DMSO (gray) are plotted with wild-type AIF monomer (red circles) and dimer (blue circles) DMSO controls. VR is calculated relative to the initial exposure of dimeric AIF–NADH. The light gray region highlights exposures containing oxidized W196A protein (t = 0.2 s, 0.4 s) before X-ray-induced FAD reduction. d, CX1 fragments stimulate binding between Atto488-labeled mitochondrial partner CHCHD4 and wild-type AIF (right) or AIF(W196A) (left). See Supplementary Table 4 for binding affinities. MST binding buffer in these experiments was supplemented with 5 mM TCEP to prevent CHCHD4 intermolecular disulfides. Each curve represents the average of three thermophoresis scans of a representative binding titration; error bars represent standard deviations.