Abstract
Background
Glioblastoma (GBM), the most common malignant brain tumor, is associated with devastating outcomes. IPAX-1 was a multicenter, open-label, single-arm phase I study to evaluate carrier-added 4-L-[131I]iodo-phenylalanine ([131I]IPA) plus external radiation therapy (XRT) in recurrent GBM.
Methods
A total of 10 adults with recurrent GBM who had received first-line debulking surgery plus radio-chemotherapy, were randomized to a single-dose regimen (1f; 131I-IPA 2 GBq before XRT); a fractionated parallel dose regimen (3f-p; 3 131I-IPA 670 MBq fractions, in parallel with second-line XRT), or a fractionated sequential dose regimen (3f-s; 3 131I-IPA 670 MBq fractions before and after XRT). Metabolic tumor responses were determined using O-(2-[18F]fluoroethyl)-l-tyrosine positron emission tomography, while single-photon emission computed tomography was used to guide [131I]IPA tumor dosimetry.
Results
All dose regimens were well tolerated. Organ-absorbed radiation doses in red marrow (0.38 Gy) and kidney (1.28 Gy) confirmed no radiation-based toxicity. Stable disease was observed in 4 of the 9 patients at 3 months post-treatment (3-month follow-up [FU], 1 patient did not reach protocol-mandated end of study), yielding a response rate of 44.4%. At the 3-month FU, 6 patients demonstrated metabolic stable disease. Median progression-free survival was 4.3 months (95% confidence interval [CI]: 3.3–4.5), while median overall survival was 13 months (95% CI: 7.1–27).
Conclusions
Single or fractionated doses of [131I]IPA plus XRT were associated with acceptable tolerability and specific tumor targeting in patients with recurrent GBM, warranting further investigation.
Keywords: amino acid transport system, glioblastoma, radiotherapy, radiopharmaceuticals, theranostics
Key Points.
The properties of [131I]IPA, radioactive effects, sustained tumor accumulation, and intrinsic cytostatic and radiosensitizing effect, make it a candidate for the treatment of gliomas.
Stable disease was achieved in 44% of patients at 3 months following [131I]IPA plus XRT treatment.
[131I]IPA plus XRT was well tolerated in patients with recurrent GBM.
Importance of the Study.
A significant unmet need exists for well-tolerated and efficacious treatments for patients with glioblastoma. The results from this phase I study demonstrated the favorable safety and tolerability profile and preliminary efficacy of 4-l-[131I]iodo-phenylalanine ([131I]IPA) in combination with second-line external radiation therapy (XRT) in patients with recurrent glioblastoma. Following treatment with [131I]IPA plus XRT, stable disease was observed in 44% (4/9) of patients at 3 months post-treatment, and median progression-free survival was 4.3 months, with no confirmed radiation toxicity. These findings support further investigation into the use of [131I]IPA plus XRT, including its potential as a first-line treatment.
Glioblastoma (GBM) is the most common form of malignant primary brain tumor and is associated with substantial morbidity and mortality.1,2 Recent advances have aided our understanding of the molecular pathogenesis and biology of these tumors,3 but this has not translated into significantly improved outcomes.2 According to the Central Brain Tumor Registry of the United States, the overall age-adjusted incidence of GBM in the United States is 3.22/100 000 persons, which increases both with advanced age at diagnosis and male sex; while the 5-year relative overall survival (OS) rate was estimated to be as low as 6.8%.1 First-line approaches to GBM therapy typically include surgery, followed by radiotherapy with concomitant temozolomide (TMZ) and TMZ maintenance therapy.3 However, standard-of-care treatments for recurrent GBM are not well defined, and treatment is typically selected based on prior therapy, age, Karnofsky Performance Score, o(6)-methylguanine-DNA methyl-transferase promoter methylation status, and patterns of disease progression.3
Amino acid transporter proteins are prime targets for imaging GBM, as they are relatively overexpressed in malignant brain tumors, including glioma cells, compared to healthy brain tissue.4 In particular, the large neutral amino acid transporter 1 (LAT1) system is overexpressed in glioma cells.5 LAT1 transports phenylalanine,6 and mediates o-(2-[18F]-fluoroethyl)-l-tyrosine ([18F]FET) uptake in gliomas, including GBM.7,8 [18F]FET positron emission tomography (PET; [18F]FET PET), allows for primary diagnosis, diagnosis of recurrence, determination of the metabolic tumor volume, in vivo grading of glioma and identification of subjects with overexpressed LAT1. LAT1 levels have also been suggested to be a prognostic marker for shorter progression-free survival (PFS) in those with gliomas.9 4-iodo-l-phenylalanine (IPA) is a derivative of the naturally occurring essential amino acid l-phenylalanine.10 Iodine-131 [131I] in [131I]IPA is cytotoxic to the cells it penetrates, alongside other cells within a several millimeter radius due to its mode of beta decay, making it a suitable therapeutic candidate for systemic, internal radiotherapy.11
The unique properties of [131I]IPA, combining preferential radioactive effects, sustained tumor accumulation, and also an intrinsic cytostatic and radiosensitizing effect, make it attractive as a candidate for glioma therapy.12 [131I]IPA was shown to influence and improve survival outcomes when combined with radiotherapy in vitro and in vivo.10,12 Furthermore, it was shown to possess the potential to histologically eradicate established experimental GBM in vivo.12 The first-in-human study with [131I]IPA as a novel treatment modality (≤6600 MBq) in 2 patients with recurrent, progressive glioma demonstrated both favorable tolerability and whole-body dosimetry, with measurable metabolic and morphological changes.13 Administration of [131I]IPA (2000–7000 MBq) with sequential XRT therapy in 5 compassionate use patients with recurrent GBM was reported to be well tolerated.14
Based on these results, [131I]IPA has been granted designated orphan status for the treatment of gliomas by the European Medicines Agency (designation no. EU/3/06/363) and the United States Food and Drug Administration (designation no. 10-3287).15 IPAX-1 was undertaken to evaluate the safety, tolerability, and preliminary efficacy of [131I]IPA in combination with second-line external radiation therapy (XRT) in patients with recurrent GBM (NCT number: NCT03849105; EudraCT number: 2018-002262-39).16,17
Materials and Methods
Study Design
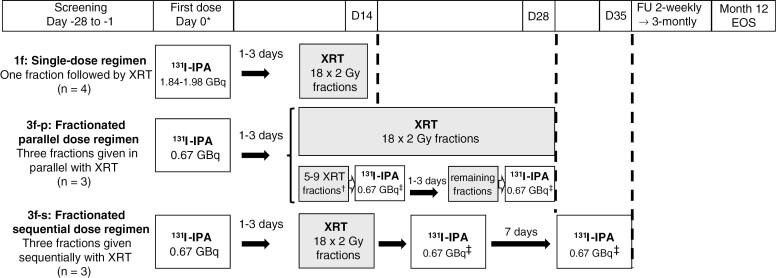
IPAX-1 was an open-label, single-arm, randomized, parallel-group, multicenter dose-finding study to evaluate the safety, tolerability, and preliminary efficacy of intravenous (IV) [131I]IPA using different dose schedules (fractionations) in combination with second-line XRT in patients with recurrent GBM (Figure 1). Screening and enrollment were conducted at 4 European centers within 28 days prior to the first treatment administration. Three dose regimen groups were assigned (Figure 1): the single dose regimen group (1f; full dose activity administered in a single fraction of 2000 MBq, followed by second-line XRT); the fractionated parallel dose regimen (3f-p; 3 670 MBq fractions administered and given in parallel with second-line XRT); and the fractionated sequential dose regimen (3f-s; 3 670 MBq fractions administered sequentially with second-line XRT; the XRT regimen started after the first [131I]IPA dose fraction was applied).
Figure 1.
Administration schedule. Abbreviations: [131I]IPA, 4-L-[131I]iodo-phenylalanine; Bq, becquerel; D, day; EOS, end of study; FU, follow-up; XRT, external radiation therapy. *Interval from the end of screening to day 0 might be ≤19 days owing to [131I]IPA manufacturing lead times; †Number of fractions subject to investigator’s discretion and depending on the day of [131I]IPA administration; ‡The second and third fractions had to be administered on the same day of the week, and some hours after administration of the respective XRT dose of that day.
Patients were not required to prepare prior to administration. To reduce non-target uptake of [131I] in the thyroid, patients were administered prophylactic sodium perchlorate or potassium iodide according to institutional standards, prior to each administration of [131I]IPA. [131I]IPA was administered on day 0 via intravenous infusion, and XRT was administered 1–3 days later. The number of fractions administered in the 3f-p and 3f-s groups was subject to the investigator’s discretion and depended on the day of [131I]IPA administration. The parallel doses of [131I]IPA administered in the 3f-p group were administered on the same day of the week, some hours after administration of the respective XRT dose of that day (Figure 1).
The mass dose for the therapeutic dose of IPA was 10 mg ± 20% at varying radioactive concentrations. This is a nominal difference throughout the spectrum of varying radioactive doses, as all doses delivered to the patient exhibit low specific activity relative to the associated cold mass content.
The calculated absorbed dose estimates of [131I]IPA within the tumor, as well as for biodistribution, were verified using both serial planar biodistribution imaging at approximately 0.5, 3, 24, and 96 hours post-injection of [131I]IPA. SPECT images were quantitatively reconstructed (qSPECT), to yield absolute activity concentrations (Bq/cm³) for tumor and normal brain (rather than conventional %ID/cm³), for all time points, to yield time activity curves (TACs), where technically feasible. For the generation of the [131I]IPA TACs, volumes of interest (VOI) were generated by manually delineating the apparent volume of pathologically increased [131I]IPA uptake, compared to normal brain tissue, in [131I]IPA SPECT/CT images. In addition, alternative VOI were generated from the baseline [18F]FET PET using 40% SUVmax as cutoff for metabolic tumor volume determination by a region-growing algorithm. PET-based VOI were fused to the [131I]IPA SPECT, using the respective co-acquired CT images of both modalities as anatomical reference. Co-localization of pathologically enhanced [131I]IPA uptake with [18F]FET PET/11C-MET PET and MRI lesions, respectively, were used to assess tumor targeting. TAC values were analyzed using IDAC2.1 to yield Gy values from [131I]IPA exposure.
[18F]FET PET scans were performed at baseline, on day 45 (± 7), day 135 (± 7), and at each 3-monthly follow-up visit from month 6 until the end of the study. Acquisition and image processing were performed according to standard institutional procedures for [18F]FET.
The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on Harmonization guideline E6: Good clinical practice. Informed consent was obtained from all individual participants included in the study.
Participants
Patients were ≥18 years of age with previously confirmed histological diagnosis of GBM with current clinical or imaging evidence for first recurrence according to modified Response Assessment in Neuro-Oncology criteria (RANO).18 Patients with recurrent GBM (World Health Organization [WHO]°IV), diagnosed in accordance with WHO classification, which required the intradialytic hypotension status, were enrolled.
Other enrollment criteria included a history of GBM standard therapy (debulking surgery, followed by radio-chemotherapy [50–60 Gy in 2 Gy fractions], and TMZ); ≥6 months since the end of first-line XRT; contrast-enhancing tumor gross tumor volume (GTV) ≤4.8 cm diameter. Re-irradiation was performed according to a fractionation scheme of 36 Gy in 18 fractions of 2 Gy. For second-line XRT, the GTV consisted of the contrast-enhancing regions on T1 sequences of the magnetic resonance image, in addition to high-uptake areas on the FET PET scans. The clinical target volume was derived from the GTV after the addition of a 0.5 cm 3D margin around the GTV and expanded to a planning target volume according to local treatment machine configuration.
Exclusion criteria included (but were not limited to) a primary XRT dose > 60 Gy; doses to organs at risk exceeded or reached by prior radiation therapy19; multifocal distant recurrence (defined as tumor lesion outside the primary XRT field, confirmed by amino acid-based PET imaging), and prior treatment with brachytherapy and/or bevacizumab. Patients who had taken any radiopharmaceutical (within a period corresponding to 8 half-lives of the radionuclide used for labeling the respective radiopharmaceutical) prior to administration of [131I]IPA were ineligible for enrollment, as were those who received any other investigational medical product (IMP) ≤90 days prior to [131I]IPA administration. Other IMPs were prohibited from the screening visit to the end of the 3-month (3-mo) follow-up (FU). During the study period, another concomitant medication was permitted at the discretion of the investigator. A full list of enrollment criteria can be found in Supplementary Information.
Study Outcomes
The primary outcomes were to assess the safety and tolerability profile of IV [131I]IPA administered concomitantly to second-line XRT in recurrent GBM. Safety investigations assessed the frequency and severity of abnormal findings (physical examination, vital signs, 12-lead electrocardiogram [ECG], clinical laboratory parameters, adverse events [AEs], and concomitant medications). Tolerability was assessed by Health-Related Quality of Life (HRQoL) total scores on the European Organization For Research And Treatment Of Cancer 30-item Quality of Life Questionnaire (EORTC QLQ-C30) and the accompanying EORTC QLQ–Brain Neoplasm (EORTC-BN20) module.20
Secondary outcomes comprised the feasibility of fractionated administration of [131I]IPA; evaluation of the radiation absorbed dose to the tumor from [131I]IPA; biodistribution and absorbed doses to whole body and organs from [131I]IPA; preliminary antineoplastic effect of [131I]IPA + second-line XRT combination therapy, and occurrence and frequency of pseudo-progression (PsPD) in response to [131I]IPA + second-line XRT combination therapy.
Study Assessments
AEs, serious AEs (SAEs), and baseline events were graded according to the National Cancer Institute Common Terminology Criteria (NCI CTCAE) version 4.03.21 By definition, for this study, all AEs were regarded as treatment emergent. AEs were further classified by preferred name and system organ class according to the Medical Dictionary for Regulatory Activities (version 25.0). Dose-limiting toxicity was defined as a grade 4 neurotoxicity or any other grade 4 toxicity according to NCI CTCAE version 4.03.21 Concomitant medications were recorded continuously from day 0 pre-dose until end of the study or treatment discontinuation. Patients underwent brain single-photon emission computed tomography (SPECT) for [131I]IPA tumor dosimetry (performed in all patients), and whole-body planar imaging for assessing [131I]IPA biodistribution and whole-body safety dosimetry. In addition, patients were comparatively assessed for possible differences in safety and/or efficacy among the different dosing regimens. Patient-determined quality of life was evaluated using the EORTC QLQ-C30 and the EORTC QLQ-BN20.20 Data were pooled by dose regimen group and analyzed according to the EORTC QLQ-C30 scoring manual, with differences over time to detect a minimum important difference of 10 points.22
Structural imaging evaluations were conducted both on-site and independently by central readers. The analysis was based on the current RANO criteria,18 considering the clinical response and use of steroids. Metabolic tumor responses with [18F]FET PET imaging were calculated by central readers from standardized uptake values to identify early or late responders to treatment. Efficacy data were collected using centralized independent evaluation, which used day 45 as a reference baseline for morphological readouts. OS was determined from day 0 (start of first [131I]IPA infusion prior to second-line XRT) to the time of death.
Results
A total of 21 patients were screened, and 10 were enrolled in the study between March 2019 and January 2021. The study was originally planned to include up to 44 patients, but recruitment was stopped after 10 patients were treated due to the sponsor’s decision as sufficient safety information was collected to progress further steps of clinical development. All 10 evaluable patients were identified as Caucasian. The mean age was 55.6 years (range 40–70 years) and 70% were male. Baseline demographics are shown in Table 1. In each of the 3f-p and 3f-s groups, 2 patients demonstrated a positive O6-methylguanine-DNA methyl-transferase promoter methylation status. All patients received 36 Gy total second-line XRT as 18 fractions of 2 Gy each, in accordance with institutional standards. The total [131I]IPA injected activity dose was 1900 ± 60 MBq in the 1f group, 1990 ± 220 MBq in the 3f-p group and 1610 ± 790 MBq in the 3f-s group (high standard deviation [SD] was owing to 1 patient only receiving 1 of the 3 dose fractions). All 10 patients were withdrawn prior to the study end (12 months): 8 were withdrawn due to disease progression and 1 was withdrawn due to an AE (platelet count decreased); and ultimately progressed within 12 months. One patient died before the study ended from acute respiratory failure caused by COVID-19. The median time from initial diagnosis through to the first day of study treatment was 10 months (range: 8–42 months; SD: 10.87 months). By the end of the study, 8 out of the 10 treated patients had died.
Table 1.
Patient Demographics and Baseline Characteristics
| Single dose 1f (N = 4) |
Fractionated dose 3f-parallel (N = 3) |
Fractionated dose 3f-sequential (N = 3) |
Total (N = 10) |
|
|---|---|---|---|---|
| Age (years) | ||||
| N | 4 | 3 | 3 | 10 |
| Median | 49 | 65 | 59 | 55 |
| Mean | 51.5 | 57.7 | 59.0 | 55.6 |
| SD | 12.5 | 13.6 | 11.0 | 11.5 |
| Minimum | 40 | 42 | 48 | 40 |
| Maximum | 69 | 66 | 70 | 70 |
| Gender | ||||
| Female | 1 (25.0%) | 1 (33.3%) | 1 (33.3%) | 3 (30.0%) |
| Male | 3 (75.0%) | 2 (66.7%) | 2 (66.7%) | 7(70.0%) |
| Race/Ethnicity | ||||
| White, non-Hispanic | 4 (100.0%) | 3 (100.0%) | 3 (100.0%) | 10 (100.0%) |
Safety and Tolerability
Adverse events.—
A total of 53 AEs were recorded (Table 2), and 8 were SAEs (Supplementary Information). The majority of treatment-emergent AEs (TEAEs; 87%) were mild or moderate in nature. As per protocol, all 28 TEAEs were considered treatment-related, of which 5 were observed in the 1f group, 20 in the 3f-p group, and 3 occurred in the same patient in the 3f-s group. A total of 4 SAEs were reported in 3 patients as possibly related to study treatment (2 events of brain edema, 1 event each of intracranial pressure increased and platelet count decreased in the same patient). One SAE that was neither related to treatment nor related to the underlying disease led to death in the 3f-p group (Supplementary Information).
Table 2.
Overview of Treatment-Emergent Adverse Events by Severity for all Patients (N = 10)
| System organ class TEAE preferred term |
Mild | Moderate | Severe | |||
|---|---|---|---|---|---|---|
| Pts, n (%) |
Events, n | Pts, n (%) |
Events, n |
Pts, n (%) |
Events, n | |
| Blood and lymphatic system disorders | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Anemia | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Ear and labyrinth disorders | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Vertigo | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Gastrointestinal disorders | 1 (10) | 1 | 1 (10) | 4 | 0 | 0 |
| Diarrhea | 1 (10) | 1 | 1 (10) | 1 | 0 | 0 |
| Gastritis | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| GERD | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Esophagitis | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| General disorders and administration site conditions | 1 (10) | 1 | 2 (20) | 2 | 1 (10) | 1 |
| Fatigue | 1 (10) | 1 | 1 (10) | 1 | 1 (10) | 1 |
| Pyrexia | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Immune system disorders | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Hypersensitivity | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Infections and infestations | 0 | 0 | 3 (30) | 3 | 0 | 0 |
| Infection | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Pneumocystis jirovecii infection | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| UTI | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Investigations | 2 (20) | 4 | 3 (30) | 3 | 2 (20) | 3 |
| Amylase increased | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Blood glucose increased | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Lipase increased | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Lymphocyte count decreased | 0 | 0 | 1 (10) | 1 | 2 (20) | 2 |
| Platelet count decreased | 0 | 0 | 1 (10) | 1 | 1 (10) | 1 |
| Weight decreased | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| WBC count decreased | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Nervous system disorders | 3 (30) | 8 | 1 (10) | 1 | 1 (10) | 1 |
| Aphasia | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Brain edema | 1 (10) | 1 | 1 (10) | 1 | 0 | 0 |
| Cognitive disorder | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Dysarthria | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Headache | 3 (30) | 3 | 0 | 0 | 0 | 0 |
| Intracranial pressure increased | 0 | 0 | 0 | 0 | 1 (10) | 1 |
| Paresthesia | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Psychiatric disorders | 1 (10) | 2 | 2 (20) | 2 | 0 | 0 |
| Agitation | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Insomnia | 1 (10) | 1 | 1 (10) | 1 | 0 | 0 |
| Sleep disorder | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | 0 | 0 | 3 (30) | 4 | 1 (10) | 1 |
| Acute respiratory failure | 0 | 0 | 0 | 0 | 1 (10) | 1 |
| Hiccups | 0 | 0 | 3 (30) | 3 | 0 | 0 |
| PE | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Skin and subcutaneous tissue disorders | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Erythema | 1 (10) | 1 | 0 | 0 | 0 | 0 |
| Vascular disorders | 0 | 0 | 1 (10) | 1 | 1 (10) | 1 |
| DVT | 0 | 0 | 1 (10) | 1 | 0 | 0 |
| Hypertension | 0 | 0 | 0 | 0 | 1 (10) | 1 |
Abbreviations: DVT, deep vein thrombosis; GERD, gastro-esophageal reflux disease; PE, pulmonary embolism; UTI, urinary tract infection; TEAE, treatment-emergent adverse event; WBC, white blood cell.
The most common TEAEs were decreased lymphocyte count, fatigue, headache, and hiccups (3 events each in 3 patients), followed by decreased platelet count, diarrhea, brain edema, and insomnia (2 events each in 2 patients). Both brain edema events were successfully treated with anti-inflammatory steroid treatment (dexamethasone), the standard of care, which is seen routinely when XRT is used. Resolution of all events occurred within days.
There were no clinically relevant or systematic changes in hematology, clinical chemistry, or coagulation during the study. Urinalysis, as well as vital signs and ECG parameters also remained unchanged and within normal parameters.
Quality of life.—
Mean HRQoL total scores decreased numerically from baseline until Day 45 and then increased again in total and in the 1f group, which was not observed in the fractionated groups (Supplementary Information).
Preliminary Efficacy and Biodistribution
Radiological response.—
Based on morphological imaging using RANO criteria, 4 of the 9 patients had stable disease at 3-month FU, yielding a response rate of 44.4%. No cases of PsPD or pseudo-response were observed in any patients.
Efficacy and dosimetry.—
Mean total body effective doses were 150.67 ± 59.01 μSv/MBq in the 1f group, 156.62 ± 53.59 μSv/MBq in the 3f-p group, and 197.00 ± 27.79 μSv/MBq in the 3f-s group.
The organs with the highest mean absorbed total dose for an assumed injected activity of 2.0 GBq were the kidneys (1f: 1.23 ± 0.43 Gy; 3f-p: 1.04 ± 0.36 Gy; 3f-s: 1.28 ± 0.26 Gy) followed by the spleen (1f: 0.89 ± 0.02 Gy; 3f-p: 0.74 ± 0.33 Gy; 3f-s: 1.24 ± 0.22 Gy) and then liver (1f: 0.47 ± 0.01 Gy; 3f-p: 0.45 ± 0.07 Gy; 3f-s: 0.56 ± 0.07 Gy). The total organ absorbed radiation dose for an assumed injected activity of 2.0 GBq for the red marrow was 0.29 ± 0.12 Gy for the 1f group, 0.30 ± 0.11 Gy for the 3f-p group, and 0.38 ± 0.06 Gy for the 3f-s group.
[131I]IPA activity mainly accumulates in the blood, liver, and spleen following excretion via the urinary system (Supplementary Information). The total mean excretion rate for [131I]IPA from the total body was 1.46 ± 0.60%/h−1 after one dose, 1.53 ± 0.72%/h−1 after 2 doses, and 1.69 ± 0.89%/h−1 after 3 doses. The mean time-integrated activity coefficients (“residence time”) were 51.87 ± 21.17 MBq*h/MBq for the 1f group, 54.62 ± 19.46 MBq*h/MBq for the 3f-p group, and 68.70 ± 10.06 MBq*h/MBq for the 3f-s group (Table 3).
Table 3.
Residence Times (TIACs) for Different Dosing Regimens of the 2000 MBq Dose Regimen Group
| Organ | 1f: Single-dose regimen Mean ± SD (MBq*h/MBq) | 3f-p: Fractionated parallel regimen Mean ± SD (MBq*h/MBq) | 3f-s: Fractionated sequential dose regimen Mean ± SD (MBq*h/MBq) |
|---|---|---|---|
| Heart contents | 2.20 ± 1.29 | 1.63 ± 0.42 | 2.27 ± 0.53 |
| Kidneys | 1.84 ± 0.64 | 1.51 ± 0.54 | 1.86 ± 0.40 |
| Liver | 3.03 ± 1.41 | 2.88 ± 0.44 | 3.57 ± 0.49 |
| Spleen | 0.70 ± 0.06 | 0.57 ± 0.27 | 0.99 ± 0.19 |
| Total body | 51.87 ± 21.17 | 54.62 ± 19.46 | 68.70 ± 10.06 |
Abbreviations: MBq, megabecquerel; SD, standard deviation; TIAC, time-integrated activity coefficient.
Analysis used (time activity curve) input from all measured source organs and the remainder of the body. [131I]IPA accumulated in the liver, kidney, spleen, urinary tract, and red marrow, being a surrogate for blood.
Metabolic tumor response.—
At the metabolic level, 6 of the 9 patients (67.7%) had stable disease based on peak uptake within the lesion, and 7 of the 9 had stable disease based on the mean lesion uptake. The metabolic tumor response was calculated based on the ratio of SUVmean and SUVpeak ratios of the tumor compared with SUVmean in the contralateral brain hemisphere. No responders were identified based on the tumor-to-brain ratio (TBR)max using a threshold of 20%. Only one patient (002-01) would have been categorized as a responder from TBRmean using a threshold of 5% at 3-mo FU. Another patient in the 3f-p cohort had a larger TBRmax than other patients and was found to have a second tumor other than glioblastoma Supplementary Information).
[131I]IPA tumor dosimetry.—
The mean normalized absorbed radiation dose delivered to the tumor was 0.70 mGy/MBq in the 1f dose regimen group, and the range of the mean normalized absorbed radiation dose per dose was 1.38–4.16 mGy/MBq in the 3f-p dose regimen group, and 0.78–1.23 mGy/MBq in the 3f-s dose regimen group (Supplementary Information). The total absorbed radiation doses ranged between 0.75–1.68 Gy in the 1f dose regimen group, 1.03–13.26 Gy in the 3f-p group, and 1.26–2.15 Gy for the 3f-s dose regimen group.
Survival outcomes.—
Median PFS was 4.3 months (95% confidence interval [CI]: 3.3–4.5 months), while median OS was 13 months (95% CI: 7.1–27 months).
Discussion
In the IPAX-1 study, patients with recurrent GBM received[131I]IPA concomitantly to second-line XRT (re-irradiation) to the tumor bed. [131I]IPA accumulates within the tumor, where it is retained exerting a low-dose rate in situ radiation. Injections of single or fractionated doses of [131I]IPA combined with second-line XRT were associated with acceptable safety and tolerability profile and no relevant laboratory changes were observed.
As expected from the underlying disease and previous treatments in these severely ill patients, neurological and hematological AEs were generally more frequent. Of 4 possibly treatment-related SAEs, there was 1 case of increased intracranial pressure and 2 cases of symptomatic brain edema, which are known events associated with radiation therapy of central nervous system cancers and were successfully treated with dexamethasone. Dexamethasone was used prophylactically at one site for 4 other patients in the study, who subsequently did not experience brain edema. Considering these observations, prophylaxis could be incorporated into this treatment regimen. Larger-scale studies are needed to assess whether the possible advantages of prophylactic dexamethasone outweigh disadvantages such as side effects (including weight gain, hyperglycemia, and myopathy)23 and the theoretical risk of decreased [131I]IPA uptake in the tumor due to normalization of the blood-tumor-barrier.24 One possibly related SAE of platelet count decrease returned to baseline levels after several months and occurred in a patient with a prior history of decreased platelet count.
To evaluate the non-target uptake of [131I]IPA, safety dosimetry evaluations were performed on all patients. While dosimetry methods have traditionally been associated with inaccuracies and poor predictive power, tumor and whole-body dosimetry has been validated in assessing the therapeutic value of [131I] meta-iodo-benzyl-guanidine (mIBG) in neuroblastoma.25 Standardized dosimetry methodology used in this study enabled precise determination of the radiation dose, allowing reliable toxicity estimation.
There were no unexpected findings regarding the impact of [131I]IPA on organ systems. Radioactive probe uptake in the lungs, heart, and thyroid were too low to acquire meaningful data. Radiation exposure to the blood (red marrow), kidney, bladder, spleen, and liver were well within tolerable limits. The total organ absorbed radiation doses were well below the dose-limiting toxicities for red marrow (2 Gy) and the kidney (23 Gy), which confirmed the lack of toxicity seen in the clinical chemistry findings. The red marrow and kidneys are known limiting factors in determining the maximum activity that can be administered. Absorbed radiation doses delivered to the kidneys were in the range of 5%, and for the red marrow at about 15% of the dose-limiting toxicities. Thus, it can be concluded that these organs are likely not at risk of damage following [131I]IPA treatment when used at the dose ranges in this study. The activity administered, 2 GBq, is within the range of conventional radioiodine-based therapy for thyroid cancer and below the lowest range used for neuroblastoma/pheochromocytoma using radioiodine labeled mIBG26,27; taken together, dosimetry results suggest that an escalation of [131I]IPA activity doses beyond 2 GBq appears feasible, confirming earlier reports.13,14
The total radiation absorbed dose range of [131I]IPA to the tumor was broad and the true preliminary efficacy impact was not feasible to measure. Imaging assessments would need to be performed on a larger population and over an expanded duration to further clarify the radiation absorbed, dose delivered, and the duration of retention of [131I]IPA within the tumor to permit treatment optimization.
Radiological and metabolic imaging was performed but indicated no significant responses. The study population was too small to draw conclusions; the treatment effect monitored with PET is not standard and must be evaluated in further studies especially when higher IPA doses are administered. As described in the literature, LAT expression in gliomas differs.9,28,29 Future studies to characterize the relationship between LAT-1 expression, FET, and IPA uptake are warranted.
The median achieved PFS of 4.3 months in IPAX-1 is similar to survival outcomes achieved previously, with reported PFS of up to 5.0 months with second-line XRT administered as a median dose of 35–36 Gy.30,31 Survival outcomes in IPAX-1 are in line with second-line studies combining XRT with bevacizumab, which have produced encouraging results, with median OS ranging from 9.7 to 38 months.32–36
There are several limitations associated with this Phase I study. A meaningful comparison cannot be made between different treatment arms due to limited patient numbers, varying injection regimens, and the early withdrawal of 1 patient after only 1 of 3 injections. Following the complete recruitment of the initial cohort, the Sponsor decided to close the study based on initial safety results indicating that [131I]IPA plus XRT was well tolerated. As XRT is an established first-line therapy for GBM, the decision was made to close this study to recruitment and commence a new study, with [131I]IPA combined with XRT in a first-line setting (NCT05450744). Given the aggressive nature of GBM and the limited lifespan of this patient population, third-line palliative therapeutic approaches in the case of clinical and/or radiological progression in most cases involve bevacizumab, which may have contributed to the survival outcomes observed. Furthermore, it cannot be excluded that certain patients received alternative therapy in the context of pseudo-progression following [131I]IPA and second-line XRT, as opposed to true progression. As the study was designed to investigate the safety of [131I]IPA, the sample sizes of each dose regimen group are too small to allow between-group statistical analysis, or to study potential clinical or histomolecular determinants of treatment effect, although we can conclude that [131I]IPA was well tolerated in this cohort.
Based on preclinical data, it is hypothesized that a combination of [131I]IPA with radiotherapy may produce an intrinsic cytostatic and radiosensitizing effect by inducing double-stranded DNA breaks in tumor cells.37 Previous evidence suggests that radiotherapy administered prior to systemic targeted radionuclide therapy can significantly increase the uptake of a radiolabeled probe.38 Conversely, if systemic radionuclide therapy is administered prior to radiotherapy, the tumoricidal effects of subsequent radiotherapy could be potentiated through tumor cell sensitization.39 It is plausible that [131I]IPA may exert a multiple mode of action consisting of the direct therapeutic effect of internalized and external radiation, which is enhanced by a cytostatic and radiosensitizer effect of IPA. Importantly, synergistic effects have also been observed with [131I]mIBG plus topotecan combination therapy in pediatric metastatic neuroblastoma, with topotecan exerting a radiosensitizing effect.40 Mechanistically, IPA, as an analog of physiological l-phenylalanine, may partially act as an antimetabolite, inhibiting DNA repair pathways between the XRT fractions, thus preventing cellular repair in the window between XRT fractions. Another advantage of this theranostic approach is employing a combination of a β and γ emitter to monitor the uptake of the substance in the target lesion, which can easily be demonstrated using SPECT imaging.
Areas for further exploration include investigating the optimal dose of [131I]IPA, the optimal sequence of internal and external radiation, the best fractionation and the best approach to re-irradiation, which all remain unknown.41 Combination of radiation with targeted drugs or immunotherapy are also potential investigative options, along with the use of different imaging markers such as radiomic features.42 Despite routine application of higher second-line XRT doses,36,43 a total radiation dose of 36 Gy was administered in IPAX-1 in 18 × 2 Gy fractions. It has been suggested that shorter fractionation might assert more benefits in terms of survival outcomes.44
The Phase I IPAX-1 study provides evidence supporting the safety and tolerability profile of [131I]IPA plus XRT combination treatment in recurrent GBM, with metabolic SD observed in 6 patients. Further refinement of the activity administration profile, and dose escalation may provide further efficacy advantages, and additional clinical studies are required to confirm these preliminary findings.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the following contributors: Ilse Vosman, MANP nurse practitioner (s’Heerenloo, The Netherlands) who performed neurological testing and supported patients throughout their care in this study and beyond; Dr Annette Leibetseder and Dr Isolde Höllmüller (Department of Neurology and Department of Internal Medicine and Neuro-oncology, Kepler Universitätsklinikum, Neuromed Campus, Linz Austria), who recruited and treated patients; Dr Robert Pichler (Department of Nuclear Medicine, Kepler Universitätsklinikum), who performed FET-PET scans; Dr Sibylle Wimmer (Department of Neuroradiology, Kepler Universitätsklinikum), who was responsible for cerebral MRI performing and RANO assessment;
Dr Serge Weis (Department of Neuropathology, Kepler Universitätsklinikum), who performed neuropathological diagnosis; Dr Friedrich Fitz (Department of Nuclear Medicine, Ordensklinikum Linz Barmherzige Schwestern, Linz, Austria), who treated the patients with the study drug;
Dr. Andreas Springer from the Department of Radiation Oncology, Ordensklinikum Linz Barmherzige Schwestern, Linz, Austria, who was the responsible physicist in treating Nuclear Medicine department; Dr. Johann Feichtinger from the Department of Radiation Oncology, Ordensklinikum Linz Barmherzige Schwestern, Linz, Austria, who was responsible for patient treatment and care during radiotherapy. Medical Writing support was provided by Health Unlimited.
Contributor Information
Josef Pichler, Department of Internal Medicine and Neuro-oncology, Kepler University Hospital, Johannes Kepler University, Linz, Austria .
Tatjana Traub-Weidinger, Division of Nuclear Medicine, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria.
Kurt Spiegl, Department of Radiation Oncology, Ordensklinikum Linz Barmherzige Schwestern, Linz, Austria.
Larisa Imamovic, Department of Nuclear Medicine, Ordensklinikum Linz Barmherzige Schwestern, Linz, Austria.
Arthur J A T Braat, Department of Radiology and Nuclear Medicine, University Medical Center Utrecht, Utrecht, The Netherlands.
Tom J Snijders, Department of Neurology, University Medical Center Utrecht, Brain Center, Utrecht, The Netherlands.
Joost J C Verhoeff, Department of Radiation Oncology, University Medical Center Utrecht, Utrecht, The Netherlands.
Patrick Flamen, Department of Nuclear Medicine, Institut Jules Bordet, Université Libre de Bruxelles, Brussels, Belgium.
Libuse Tauchmanova, TelixPharmaceuticals, North Melbourne, VIC, Australia.
Colin Hayward, TelixPharmaceuticals, North Melbourne, VIC, Australia.
Andreas Kluge, ABX - CRO Advanced Pharmaceutical Services Forschungsgesellschaft, Dresden, Germany.
Funding
This work was supported by TELIX Pharmaceuticals.
Conflict of interest statement
K. Spiegl, L. Imamovic, A.J.A.T. Braat, J.J.C. Verhoeff, T. Traub-Weidinger and T. Snijders: No competing interests. J. Pichler has received consultant honoraria and research support for performing scientific research from Telix Pharmaceuticals. L. Tauchmanova and C. Hayward are employees of Telix Pharmaceuticals. A. Kluge is founder and shareholder of Telix Pharmaceuticals. TJ Schnijders, JJC Verhoeff: The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Authorship statement
All authors made substantial contributions to the conception or design of this work and to the analysis and interpretation of data. All authors were involved in drafting the manuscript and subsequent revising of the content and provided final approval of the published version.
References
- 1. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS.. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021;23(12 suppl 2):iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen L, Ma J, Zou Z, et al. Clinical characteristics and prognosis of patients with glioblastoma: A review of survival analysis of 1674 patients based on SEER database. Medicine (Baltim). 2022;101(47):e32042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Santo G, Laudicella R, Linguanti F, et al. The utility of conventional amino acid PET radiotracers in the evaluation of glioma recurrence also in comparison with MRI. Diagnostics (Basel). 2022;12(4):844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Habermeier A, Graf J, Sandhofer BF, et al. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET). Amino Acids. 2015;47(2):335–344. [DOI] [PubMed] [Google Scholar]
- 6. Zhao Y, Wang L, Pan J.. The role of L-type amino acid transporter 1 in human tumors. Intractable Rare Dis Res. 2015;4(4):165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cai L, Kirchleitner SV, Zhao D, et al. Glioblastoma exhibits inter-individual heterogeneity of TSPO and LAT1 expression in neoplastic and parenchymal cells. Int J Mol Sci . 2020;21(2):612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Popperl G, Kreth FW, Herms J, et al. Analysis of 18F-FET PET for grading of recurrent gliomas: Is evaluation of uptake kinetics superior to standard methods? J Nucl Med. 2006;47(3):393–403. [PubMed] [Google Scholar]
- 9. Vettermann FJ, Diekmann C, Weidner L, et al. L-type amino acid transporter (LAT) 1 expression in (18)F-FET-negative gliomas. EJNMMI Res. 2021;11(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romeike BF, Hellwig D, Heimann A, et al. Action and efficacy of p-[131I]iodo-L-phenylalanine on primary human glioma cell cultures and rats with C6-gliomas. Anticancer Res. 2004;24(6):3971–3976. [PubMed] [Google Scholar]
- 11. Petrov SA, Yusubov MS, Beloglazkina EK, Nenajdenko VG.. Synthesis of radioiodinated compounds. Classical approaches and achievements of recent years. Int J Mol Sci . 2022;23(22):13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samnick S, Romeike BF, Lehmann T, et al. Efficacy of systemic radionuclide therapy with p-131I-iodo-L-phenylalanine combined with external beam photon irradiation in treating malignant gliomas. J Nucl Med. 2009;50(12):2025–2032. [DOI] [PubMed] [Google Scholar]
- 13. Baum RP, Kluge A, Gildehaus FJ, et al. Systemic endoradiotherapy with carrier-added 4-[(131)I]iodo-L-phenylalanine: Clinical proof-of-principle in refractory glioma. Nucl Med Mol Imaging. 2011;45(4):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verburg FA, Sweeney R, Hanscheid H, et al. Patients with recurrent glioblastoma multiforme. Initial experience with p-[(131)I]iodo-L-phenylalanine and external beam radiation therapy. Nuklearmedizin. 2013;52(1):36–42. [DOI] [PubMed] [Google Scholar]
- 15. European Medicines Agency. EU/3/06/363: Orphan designation for the treatment of glioma. https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu306363 [Google Scholar]
- 16. ClinicalTrials.gov. NCT03849105. 131I-IPA and Concurrent XRT in Recurrent GBM (IPAX-1). https://clinicaltrials.gov/ct2/show/NCT03849105 [Google Scholar]
- 17. Register ECT. EudraCT number 2018-002262-39. https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-002262-39/AT [Google Scholar]
- 18. Ellingson BM, Wen PY, Cloughesy TF.. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yasar D, Tugrul AB.. A new approach for the calculation of critical organ dose in nuclear medicine applications. Appl Radiat Isot. 2005;62(3):405–410. [DOI] [PubMed] [Google Scholar]
- 20. EORTC Quality of Life. Questionnaires. BN20 update. https://qol.eortc.org/questionnaire/bn20-update/ [Google Scholar]
- 21. National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009; v4.03: June 14, 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf [Google Scholar]
- 22. Osoba D, Rodrigues G, Myles J, Zee B, Pater J.. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. [DOI] [PubMed] [Google Scholar]
- 23. Dietrich J, Rao K, Pastorino S, Kesari S.. Corticosteroids in brain cancer patients: Benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4(2):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilkinson ID, Jellineck DA, Levy D, et al. Dexamethasone and enhancing solitary cerebral mass lesions: Alterations in perfusion and blood-tumor barrier kinetics shown by magnetic resonance imaging. Neurosurgery. 2006;58(4):640–6; discussion 640. [DOI] [PubMed] [Google Scholar]
- 25. Matthay KK, Panina C, Huberty J, et al. Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I-MIBG. J Nucl Med. 2001;42(11):1713–1721. [PubMed] [Google Scholar]
- 26. Giammarile F, Chiti A, Lassmann M, Brans B, Flux G; Eanm. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur J Nucl Med Mol Imaging. 2008;35(5):1039–1047. [DOI] [PubMed] [Google Scholar]
- 27. Silberstein EB, Alavi A, Balon HR, et al. The SNMMI practice guideline for therapy of thyroid disease with 131I 3.0. J Nucl Med. 2012;53(10):1633–1651. [DOI] [PubMed] [Google Scholar]
- 28. Haining Z, Kawai N, Miyake K, et al. Relation of LAT1/4F2hc expression with pathological grade, proliferation and angiogenesis in human gliomas. BMC Clin Pathol. 2012;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nawashiro H, Otani N, Shinomiya N, et al. L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int J Cancer. 2006;119(3):484–492. [DOI] [PubMed] [Google Scholar]
- 30. Combs SE, Gutwein S, Thilmann C, et al. Stereotactically guided fractionated re-irradiation in recurrent glioblastoma multiforme. J Neurooncol. 2005;74(2):167–171. [DOI] [PubMed] [Google Scholar]
- 31. Breen WG, Youland RS, Giri S, et al. Initial results of a phase II trial of (18)F-DOPA PET-guided re-irradiation for recurrent high-grade glioma. J Neurooncol. 2022;158(3):323–330. [DOI] [PubMed] [Google Scholar]
- 32. Schernberg A, Dhermain F, Ammari S, et al. Reirradiation with concurrent bevacizumab for recurrent high-grade gliomas in adult patients. Cancer Radiother. 2018;22(1):9–16. [DOI] [PubMed] [Google Scholar]
- 33. Fleischmann DF, Jenn J, Corradini S, et al. Bevacizumab reduces toxicity of reirradiation in recurrent high-grade glioma. Radiother Oncol. 2019;138:99–105. [DOI] [PubMed] [Google Scholar]
- 34. Palmer JD, Bhamidipati D, Song A, et al. Bevacizumab and re-irradiation for recurrent high grade gliomas: Does sequence matter? J Neurooncol. 2018;140(3):623–628. [DOI] [PubMed] [Google Scholar]
- 35. Back M, Gzell CE, Kastelan M, Guo L, Wheeler HR.. Large volume re-irradiation with bevacizumab is a feasible salvage option for patients with refractory high-grade glioma. Neurooncol. Pract.. 2015;2(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Post CCB, Kramer MCA, Smid EJ, et al. Patterns of re-irradiation for recurrent gliomas and validation of a prognostic score. Radiother Oncol. 2019;130:156–163. [DOI] [PubMed] [Google Scholar]
- 37. Neshasteh-Riz A, Koosha F, Mohsenifar A, Mahdavi SR.. DNA Damage induced in glioblastoma cells by I-131: A comparison between experimental data and monte carlo simulation. Cell J. 2012;14(1):25–30. [PMC free article] [PubMed] [Google Scholar]
- 38. Msirikale JS, Klein JL, Schroeder J, Order SE.. Radiation enhancement of radiolabelled antibody deposition in tumors. Int J Radiat Oncol Biol Phys. 1987;13(12):1839–1844. [DOI] [PubMed] [Google Scholar]
- 39. Knox SJ, Sutherland W, Goris ML.. Correlation of tumor sensitivity to low-dose-rate irradiation with G2/M-phase block and other radiobiological parameters. Radiat Res. 1993;135(1):24–31. [PubMed] [Google Scholar]
- 40. Gaze MN, Chang YC, Flux GD, et al. Feasibility of dosimetry-based high-dose 131I-meta-iodobenzylguanidine with topotecan as a radiosensitizer in children with metastatic neuroblastoma. Cancer Biother Radiopharm. 2005;20(2):195–199. [DOI] [PubMed] [Google Scholar]
- 41. Garcia-Cabezas S, Rivin Del Campo E, Solivera-Vela J, Palacios-Eito A.. Rivin Del Campo E, Solivera-Vela J, Palacios-Eito A. Re-irradiation for high-grade gliomas: Has anything changed? World J Clin Oncol. 2021;12(9):767–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fornacon-Wood I, Mistry H, Ackermann CJ, et al. Reliability and prognostic value of radiomic features are highly dependent on choice of feature extraction platform. Eur Radiol. 2020;30(11):6241–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nieder C, Andratschke NH, Grosu AL.. Re-irradiation for recurrent primary brain tumors. Anticancer Res. 2016;36(10):4985–4995. [DOI] [PubMed] [Google Scholar]
- 44. Kazmi F, Soon YY, Leong YH, Koh WY, Vellayappan B.. Re-irradiation for recurrent glioblastoma (GBM): A systematic review and meta-analysis. J Neurooncol. 2019;142(1):79–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.