Abstract
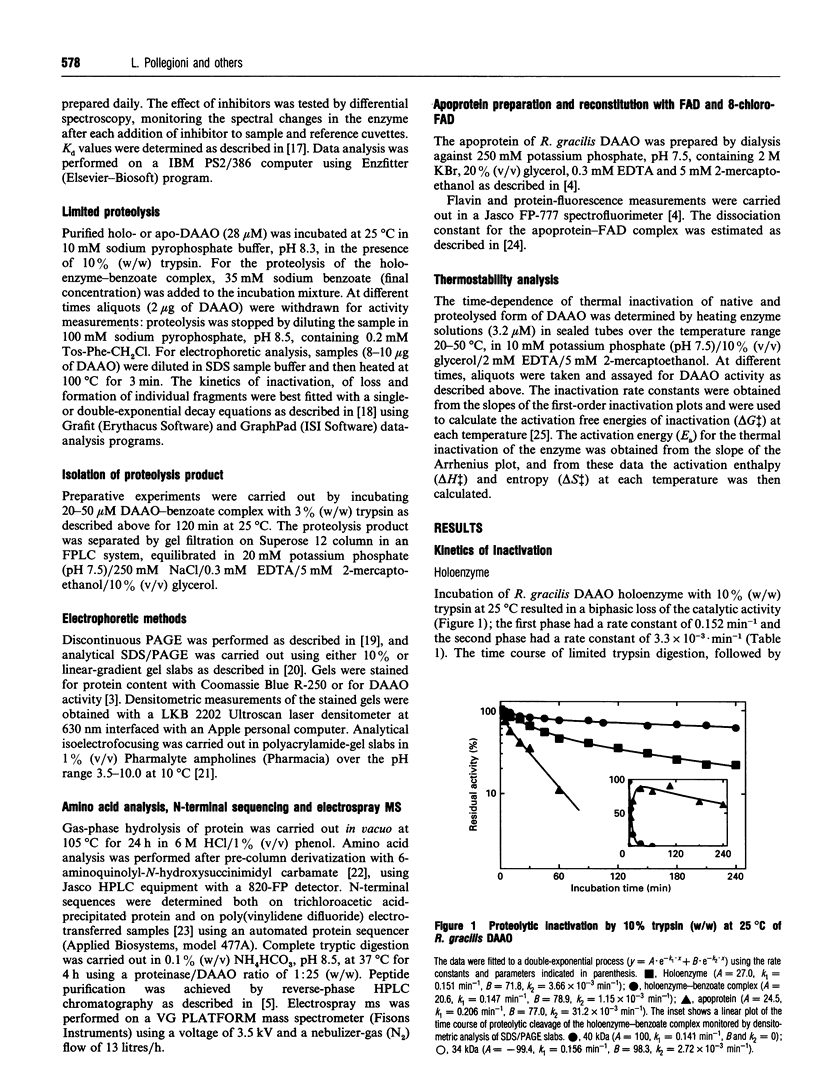
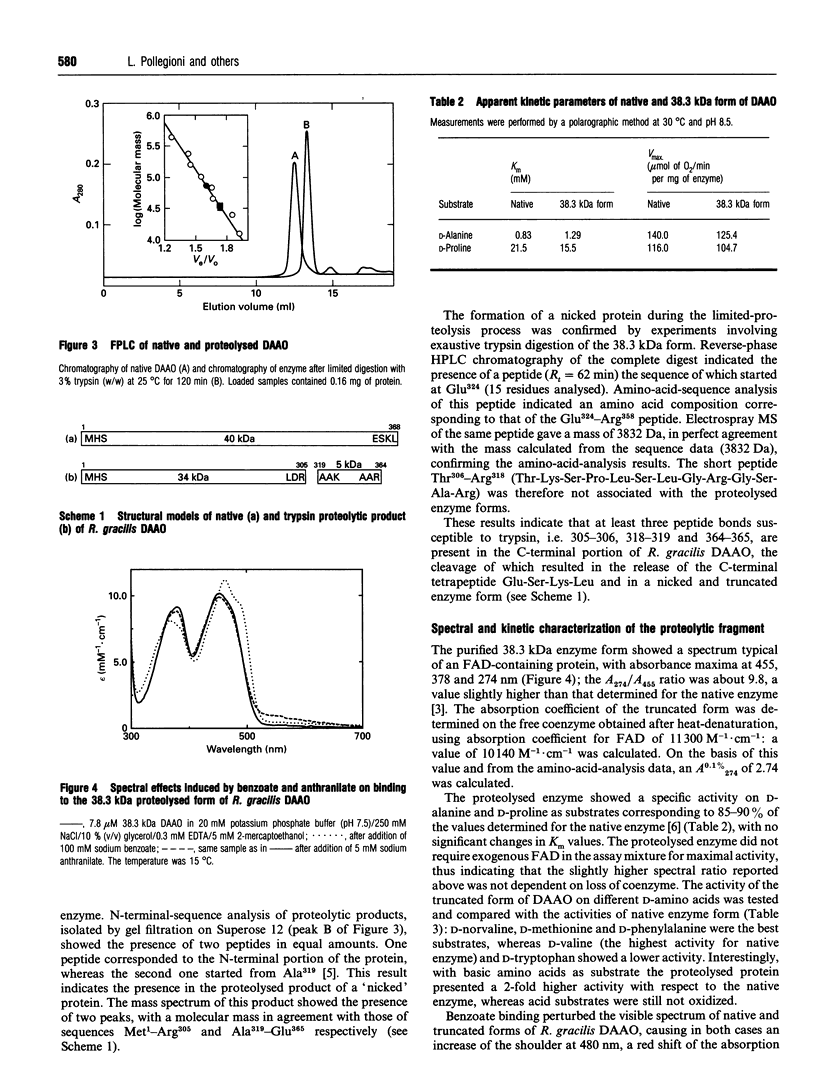
The structure-function relationships of purified Rhodotorula gracilis D-amino acid oxidase (in its holo-, apo- and holo-enzyme-benzoate complex forms) was analysed by digestion with trypsin. In all cases trypsin cleaves this 80 kDa dimeric enzyme at the C-terminal region, since the peptide bonds sensitive to proteinase attack are clustered in this region. Digestion of native enzyme with trypsin produced a nicked and truncated form of 38.3 kDa containing two polypeptides of 34 and 5 kDa starting from Met1 and Ala319 respectively, and with detachment of the Thr306-Arg318 and Glu365-Leu368 peptides. Our results show that this 'core', folded into a compact structure, is catalytically competent. The acquisition of this nicked form was marked by a shift from a dimeric to a monomeric active enzyme, a result never previously obtained. The deleted sequences, Thr306-Arg318 and Glu365-Leu368, are essential for the monomer-monomer interaction, and, in particular, the region encompassing Thr306-Arg318 should play an essential role in the dimerization process. interestingly, the Ser308-Lys321 sequence present in the lost peptide corresponds to a sequence not present in other known D-amino acid oxidases [Faotto, Pollegioni, Ceciliani, Ronchi and Pilone (1995) Biotechnol. Lett. 17, 193-198]. A role of the cleaved-off region for the thermostabilization of the enzyme is also discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casalin P., Pollegioni L., Curti B., Pilone Simonetta M. A study on apoenzyme from Rhodotorula gracilis D-amino acid oxidase. Eur J Biochem. 1991 Apr 23;197(2):513–517. doi: 10.1111/j.1432-1033.1991.tb15939.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Michaud D. P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem. 1993 Jun;211(2):279–287. doi: 10.1006/abio.1993.1270. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dill K. A. Dominant forces in protein folding. Biochemistry. 1990 Aug 7;29(31):7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- Fonda M. L., Anderson B. M. D-amino acid oxidase. I. Spectrophotometric studies. J Biol Chem. 1967 Sep 10;242(17):3957–3962. [PubMed] [Google Scholar]
- Gadda G., Beretta G. L., Pilone M. S. Chemical modification of lysyl residues of Rhodotorula gracilis D-amino acid oxidase. Biochem Mol Biol Int. 1994 Aug;33(5):947–955. [PubMed] [Google Scholar]
- Gadda G., Negri A., Pilone M. S. Reaction of phenylglyoxal with arginine groups in D-amino-acid oxidase from Rhodotorula gracilis. J Biol Chem. 1994 Jul 8;269(27):17809–17814. [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Subramani S. Identification of peroxisomal targeting signals located at the carboxy terminus of four peroxisomal proteins. J Cell Biol. 1988 Sep;107(3):897–905. doi: 10.1083/jcb.107.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. T., Richardson C. C. Acidic carboxyl-terminal domain of gene 2.5 protein of bacteriophage T7 is essential for protein-protein interactions. J Biol Chem. 1994 Feb 18;269(7):5270–5278. [PubMed] [Google Scholar]
- Kristjansson M. M., Kinsella J. E. Alkaline serine proteinase from Thermomonospora fusca YX. Stability to heat and denaturants. Biochem J. 1990 Aug 15;270(1):51–55. doi: 10.1042/bj2700051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSEY V., GIBSON Q. H. ROLE OF SEMIQUINONES IN FLAVOPROTEIN CATALYSIS. Fed Proc. 1964 Jan-Feb;23:18–29. [PubMed] [Google Scholar]
- Massey V., Ganther H. On the interpretation of the absorption spectra of flavoproteins with special reference to D-amino acid oxidase. Biochemistry. 1965 Jun;4(6):1161–1173. doi: 10.1021/bi00882a027. [DOI] [PubMed] [Google Scholar]
- Massey V., Hemmerich P. Active-site probes of flavoproteins. Biochem Soc Trans. 1980 Jun;8(3):246–257. doi: 10.1042/bst0080246. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Moore E. G., Ghisla S., Massey V. Properties of flavins where the 8-methyl group is replaced by mercapto- residues. J Biol Chem. 1979 Sep 10;254(17):8173–8178. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pilone Simonetta M., Pollegioni L., Casalin P., Curti B., Ronchi S. Properties of D-amino-acid oxidase from Rhodotorula gracilis. Eur J Biochem. 1989 Mar 1;180(1):199–204. doi: 10.1111/j.1432-1033.1989.tb14634.x. [DOI] [PubMed] [Google Scholar]
- Pollegioni L., Falbo A., Pilone M. S. Specificity and kinetics of Rhodotorula gracilis D-amino acid oxidase. Biochim Biophys Acta. 1992 Mar 27;1120(1):11–16. doi: 10.1016/0167-4838(92)90418-d. [DOI] [PubMed] [Google Scholar]
- Pollegioni L., Ghisla S., Pilone M. S. Studies on the active centre of Rhodotorula gracilis D-amino acid oxidase and comparison with pig kidney enzyme. Biochem J. 1992 Sep 1;286(Pt 2):389–394. doi: 10.1042/bj2860389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollegioni L., Langkau B., Tischer W., Ghisla S., Pilone M. S. Kinetic mechanism of D-amino acid oxidases from Rhodotorula gracilis and Trigonopsis variabilis. J Biol Chem. 1993 Jul 5;268(19):13850–13857. [PubMed] [Google Scholar]
- Pollegioni L., Pilone M. S. Purification of Rhodotorula gracilis D-amino acid oxidase. Protein Expr Purif. 1992 Apr;3(2):165–167. [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr A method for characterizing the type and numbers of groups involved in enzyme action. J Biol Chem. 1961 Jul;236:1973–1979. [PubMed] [Google Scholar]
- Ronchi S., Minchiotti L., Galliano M., Curti B., Swenson R. P., Williams C. H., Jr, Massey V. The primary structure of D-amino acid oxidase from pig kidney. II. Isolation and sequence of overlap peptides and the complete sequence. J Biol Chem. 1982 Aug 10;257(15):8824–8834. [PubMed] [Google Scholar]
- Schräder T., Andreesen J. R. Evidence for the functional importance of Cys298 in D-amino acid oxidase from Trigonopsis variabilis. Eur J Biochem. 1993 Dec 1;218(2):735–744. doi: 10.1111/j.1432-1033.1993.tb18428.x. [DOI] [PubMed] [Google Scholar]
- Stinson R. A., Holbrook J. J. Equilibrium binding of nicotinamide nucleotides to lactate dehydrogenases. Biochem J. 1973 Apr;131(4):719–728. doi: 10.1042/bj1310719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berghe-Snorek S., Stankovich M. T. Thermodynamic control of D-amino acid oxidase by benzoate binding. J Biol Chem. 1985 Mar 25;260(6):3373–3379. [PubMed] [Google Scholar]
- Watanabe F., Fukui K., Momoi K., Miyake Y. Effect of site-specific mutagenesis of tyrosine-55, methionine-110 and histidine-217 in porcine kidney D-amino acid oxidase on its catalytic function. FEBS Lett. 1988 Oct 10;238(2):269–272. doi: 10.1016/0014-5793(88)80494-0. [DOI] [PubMed] [Google Scholar]
- Xia Z. X., Mathews F. S. Molecular structure of flavocytochrome b2 at 2.4 A resolution. J Mol Biol. 1990 Apr 20;212(4):837–863. doi: 10.1016/0022-2836(90)90240-M. [DOI] [PubMed] [Google Scholar]



