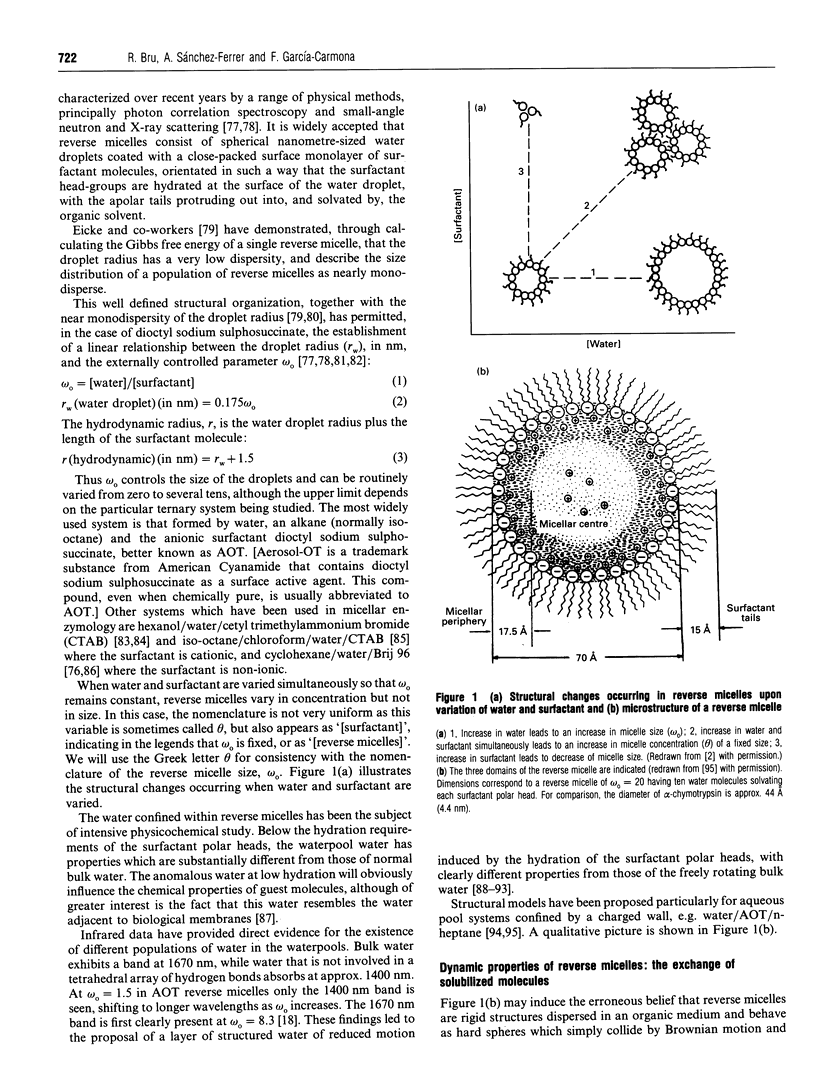
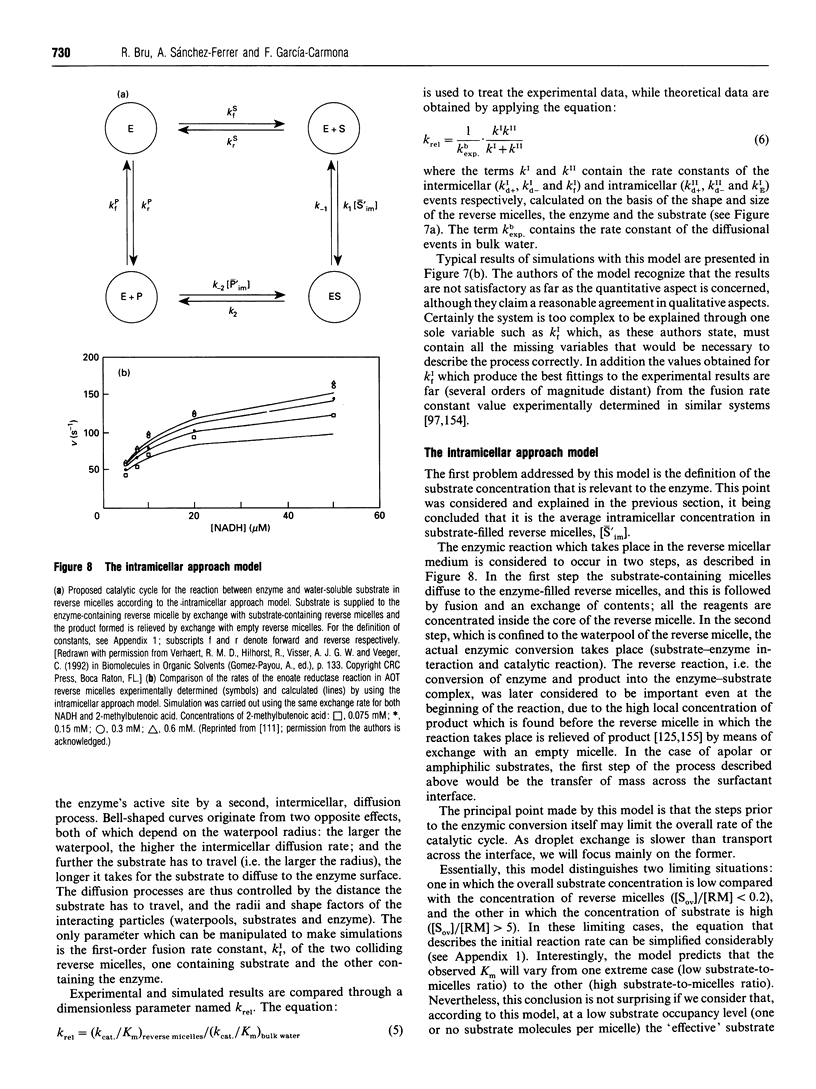
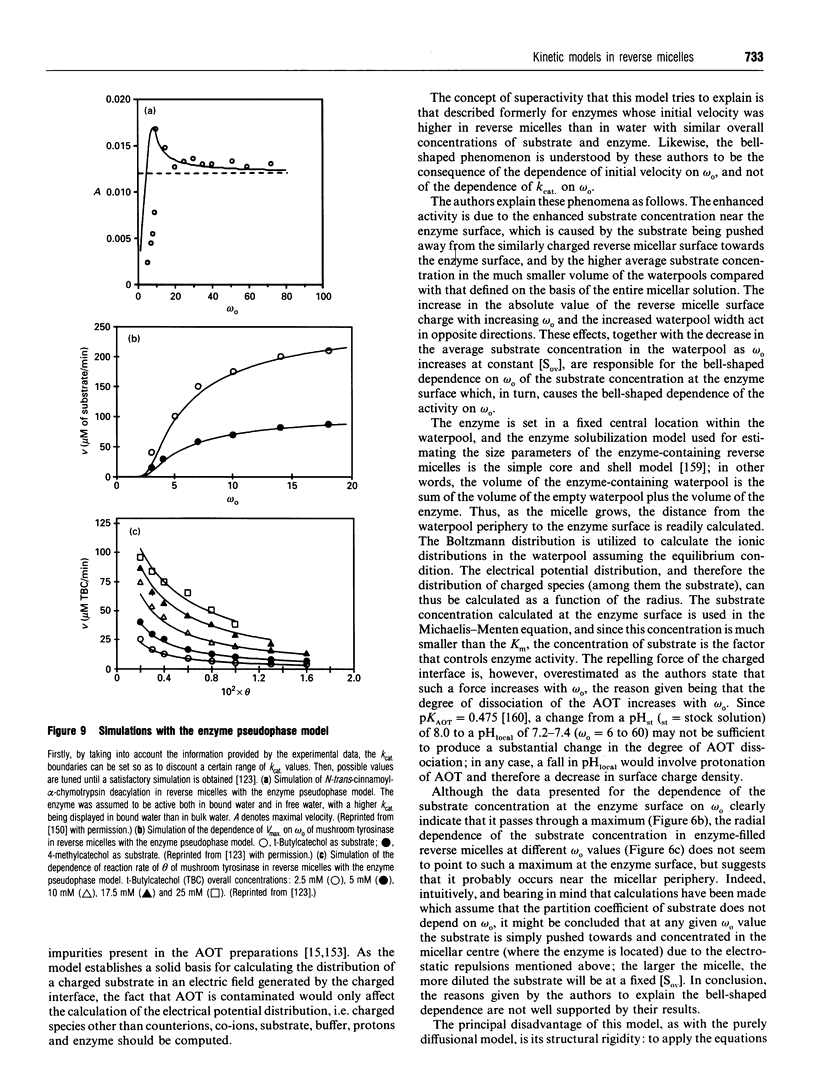
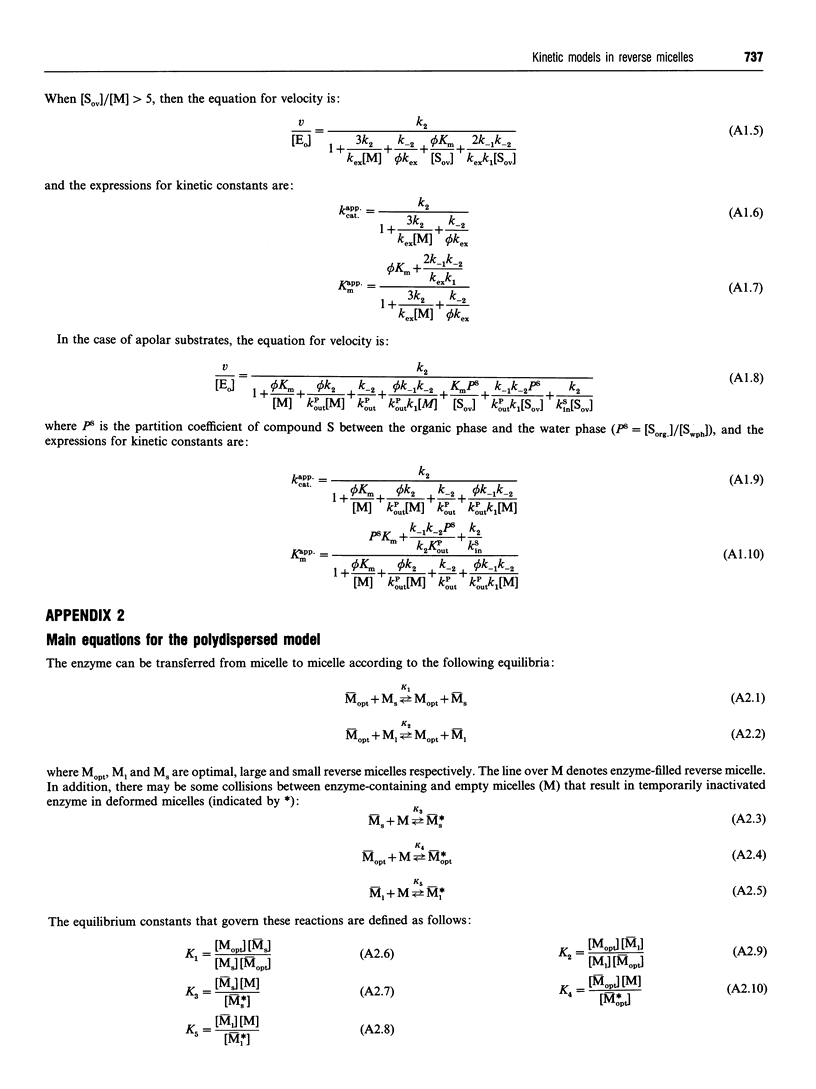
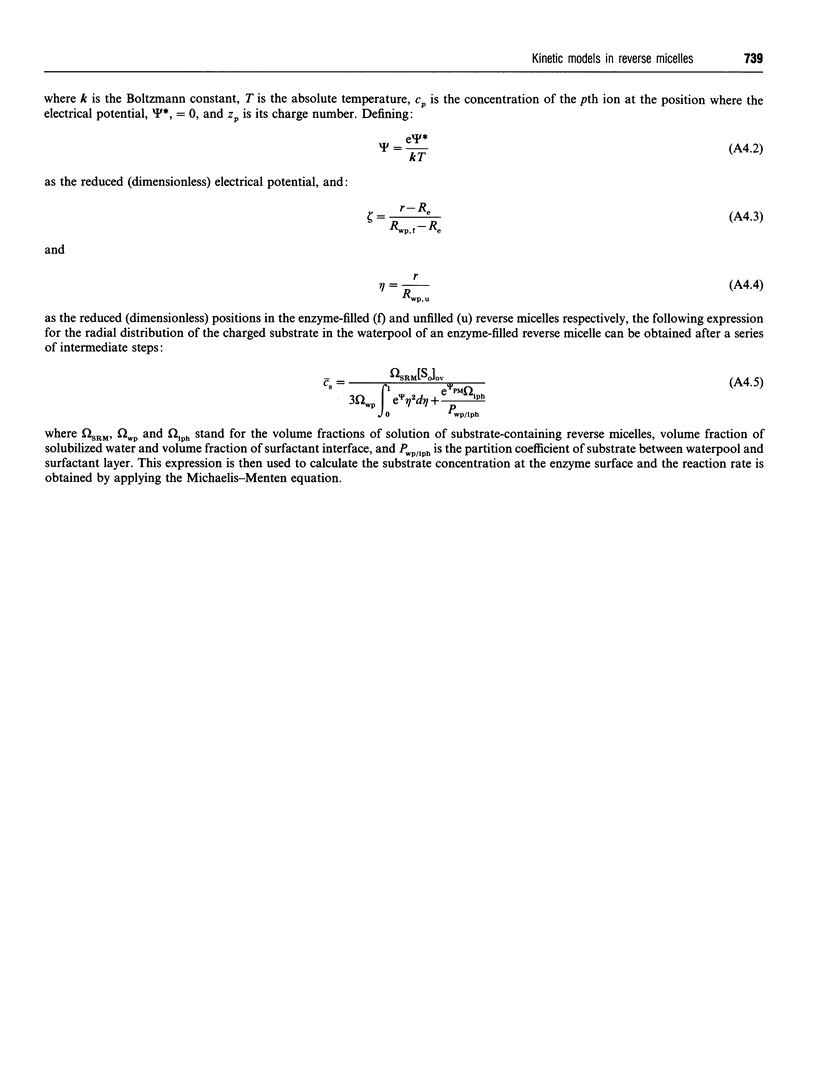
Full text
PDF
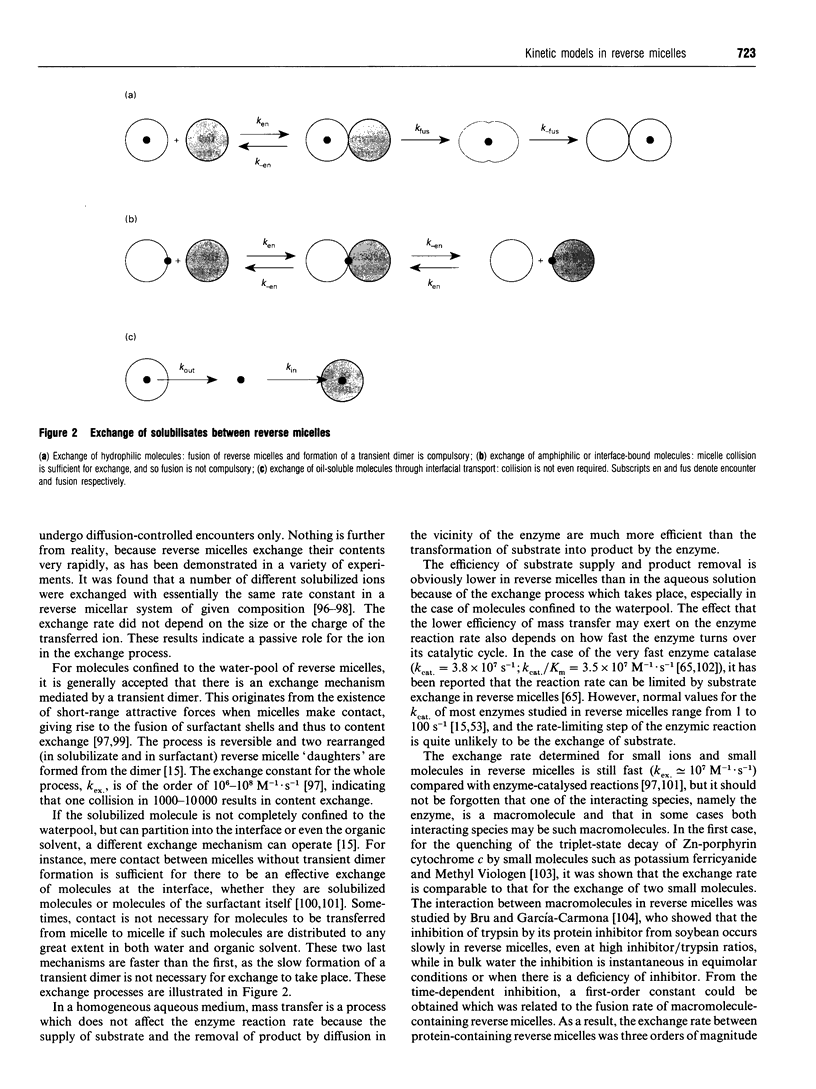



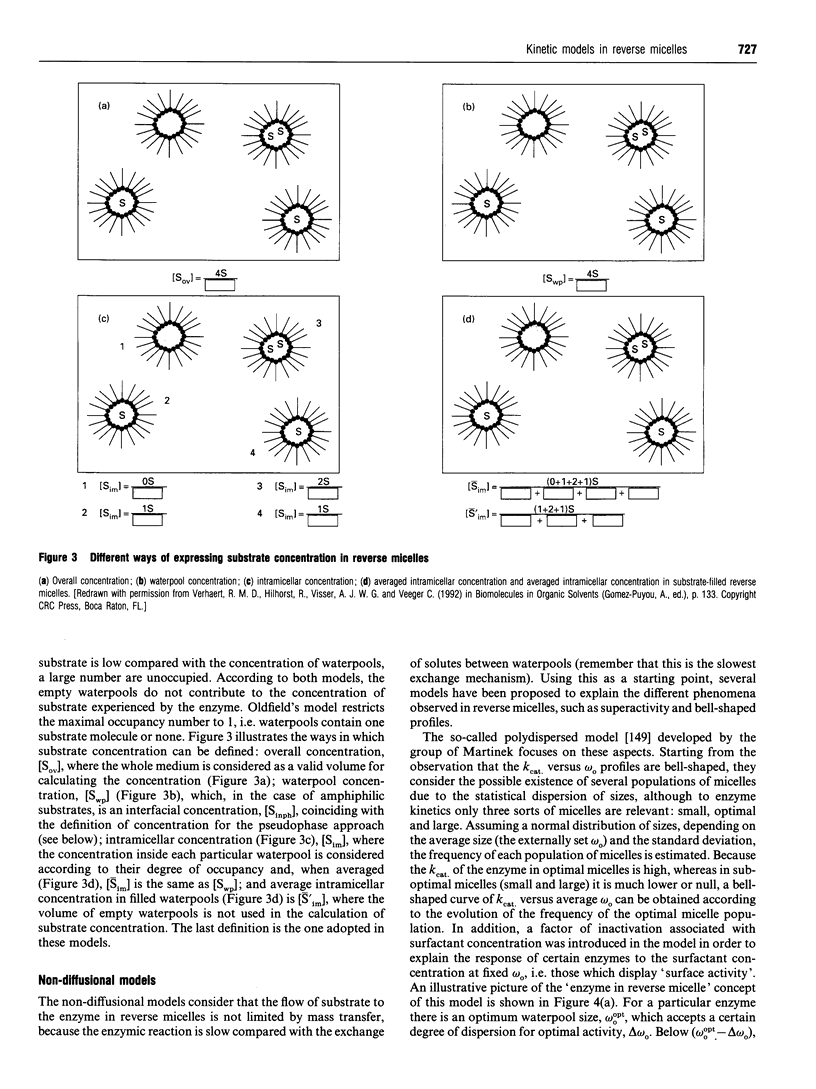
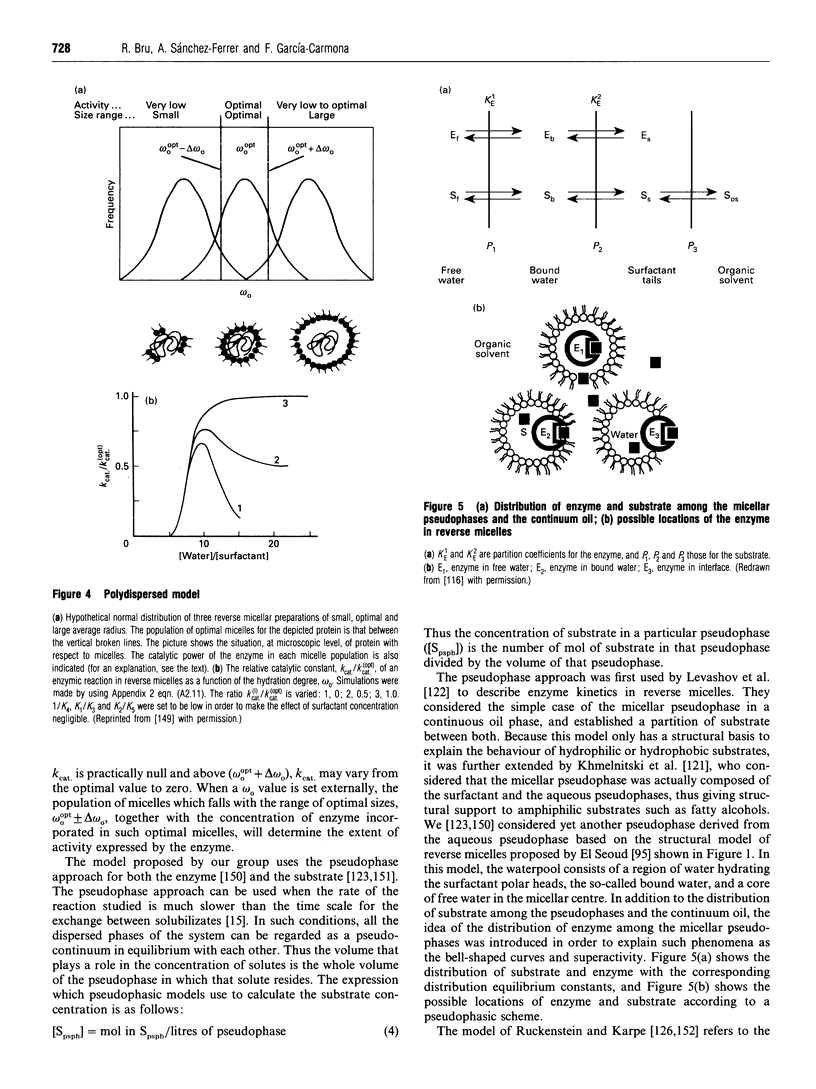
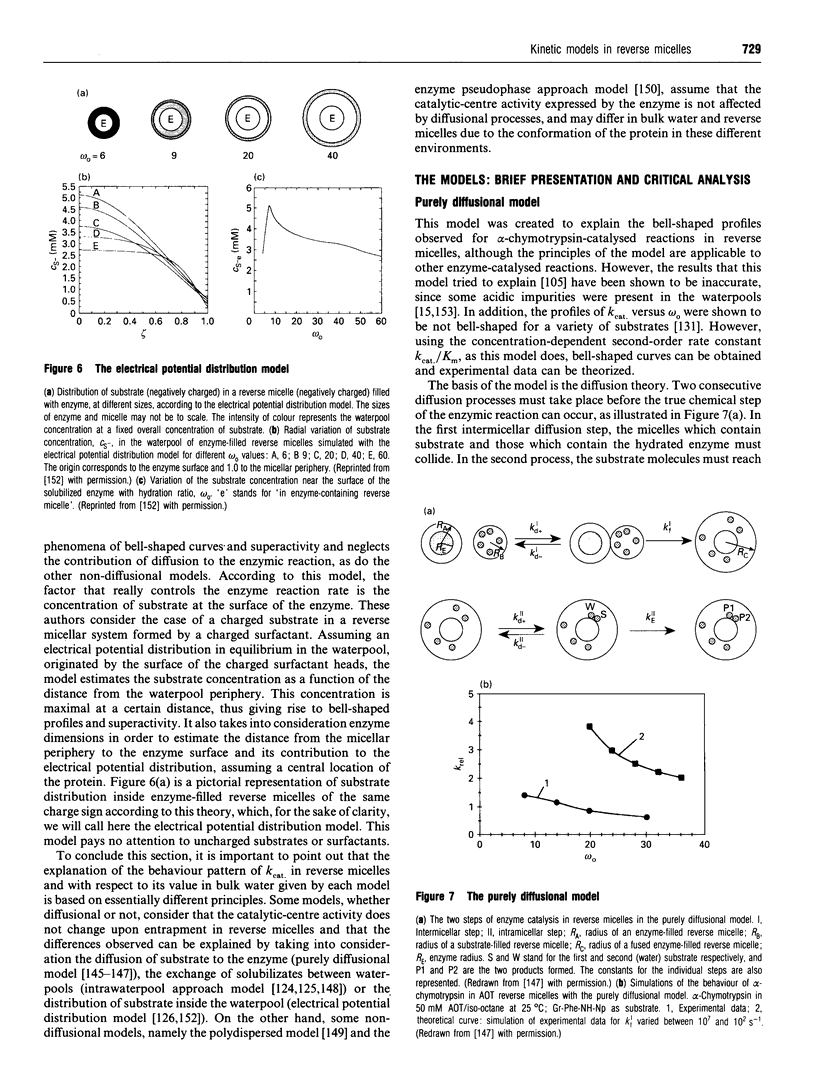






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bru R., García-Carmona F. Trypsin-SBTI interaction in reverse micelles. A slow intermicellar exchange-dependent binding. FEBS Lett. 1991 Apr 22;282(1):170–174. doi: 10.1016/0014-5793(91)80470-n. [DOI] [PubMed] [Google Scholar]
- Bru R., Sanchez-Ferrer A., García-Carmona F. Characteristics of tyrosinase in AOT-isooctane reverse micelles. Biotechnol Bioeng. 1989 Jul;34(3):304–308. doi: 10.1002/bit.260340305. [DOI] [PubMed] [Google Scholar]
- Bru R., Sánchez-Ferrer A., Garcia-Carmona F. A theoretical study on the expression of enzymic activity in reverse micelles. Biochem J. 1989 Apr 15;259(2):355–361. doi: 10.1042/bj2590355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru R., Sánchez-Ferrer A., García-Carmona F. The effect of substrate partitioning on the kinetics of enzymes acting in reverse micelles. Biochem J. 1990 Jun 15;268(3):679–684. doi: 10.1042/bj2680679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru R., Walde P. Catalytic activity of elastase in reverse micelles. Biochem Mol Biol Int. 1993 Nov;31(4):685–692. [PubMed] [Google Scholar]
- Bru R., Walde P. Product inhibition of alpha-chymotrypsin in reverse micelles. Eur J Biochem. 1991 Jul 1;199(1):95–103. doi: 10.1111/j.1432-1033.1991.tb16096.x. [DOI] [PubMed] [Google Scholar]
- Chatenay D., Urbach W., Cazabat A. M., Vacher M., Waks M. Proteins in membrane mimetic systems. Insertion of myelin basic protein into microemulsion droplets. Biophys J. 1985 Dec;48(6):893–898. doi: 10.1016/S0006-3495(85)83851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. S. Intracellular water and the cytomatrix: some methods of study and current views. J Cell Biol. 1984 Jul;99(1 Pt 2):167s–171s. doi: 10.1083/jcb.99.1.167s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Kuntz I. D. The properties of water in biological systems. Annu Rev Biophys Bioeng. 1974;3(0):95–126. doi: 10.1146/annurev.bb.03.060174.000523. [DOI] [PubMed] [Google Scholar]
- Dekker M., Hilhorst R., Laane C. Isolating enzymes by reversed micelles. Anal Biochem. 1989 May 1;178(2):217–226. doi: 10.1016/0003-2697(89)90628-3. [DOI] [PubMed] [Google Scholar]
- Delahodde A., Vacher M., Nicot C., Waks M. Solubilization and insertion into reverse micelles of the major myelin transmembrane proteolipid. FEBS Lett. 1984 Jul 9;172(2):343–347. doi: 10.1016/0014-5793(84)81154-0. [DOI] [PubMed] [Google Scholar]
- Dorovska-Taran V. N., Veeger C., Visser A. J. Comparison of the dynamic structure of alpha-chymotrypsin in aqueous solution and in reversed micelles by fluorescent active-site probing. Eur J Biochem. 1993 Jan 15;211(1-2):47–55. doi: 10.1111/j.1432-1033.1993.tb19868.x. [DOI] [PubMed] [Google Scholar]
- Drost-Hansen W. Phase transitions in biological systems: manifestations of cooperative processes in vicinal water. Ann N Y Acad Sci. 1973 Mar 30;204:100–112. doi: 10.1111/j.1749-6632.1973.tb30773.x. [DOI] [PubMed] [Google Scholar]
- Erjomin A. N., Metelitza D. I. Catalysis by hemoproteins and their structural organization in reversed micelles of surfactants in octane. Biochim Biophys Acta. 1983 Jul 27;732(2):377–386. doi: 10.1016/0005-2736(83)90054-8. [DOI] [PubMed] [Google Scholar]
- Escamilla E., Ayala G., de Gómez-Puyou M. T., Gómez-Puyou A., Millán L., Darszon A. Catalytic activity of cytochrome oxidase and cytochrome c in apolar solvents containing phospholipids and low amounts of water. Arch Biochem Biophys. 1989 Aug 1;272(2):332–343. doi: 10.1016/0003-9861(89)90227-0. [DOI] [PubMed] [Google Scholar]
- Garza-Ramos G., Darszon A., Tuena de Gómez-Puyou M., Gómez-Puyou A. Catalysis and thermostability of mitochondrial F1-ATPase in toluene-phospholipid-low-water systems. Biochemistry. 1989 Apr 18;28(8):3177–3182. doi: 10.1021/bi00434a010. [DOI] [PubMed] [Google Scholar]
- Garza-Ramos G., Darszon A., Tuena de Gómez-Puyou M., Gómez-Puyou A. Enzyme catalysis in organic solvents with low water content at high temperatures. The adenosinetriphosphatase of submitochondrial particles. Biochemistry. 1990 Jan 23;29(3):751–757. doi: 10.1021/bi00455a023. [DOI] [PubMed] [Google Scholar]
- Gierasch L. M., Lacy J. E., Thompson K. F., Rockwell A. L., Watnick P. I. Conformations of model peptides in membrane-mimetic environments. Biophys J. 1982 Jan;37(1):275–284. doi: 10.1016/S0006-3495(82)84676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi C., Smith R. E., Luisi P. L. Micellar solubilization of biopolymers in organic solvents. Activity and conformation of lysozyme in isooctane reverse micelles. J Biol Chem. 1981 Jan 25;256(2):837–843. [PubMed] [Google Scholar]
- Haber J., Maślakiewicz P., Rodakiewicz-Nowak J., Walde P. Activity and spectroscopic properties of bovine liver catalase in sodium bis(2-ethylhexyl)sulfosuccinate/isooctane reverse micelles. Eur J Biochem. 1993 Oct 15;217(2):567–573. doi: 10.1111/j.1432-1033.1993.tb18278.x. [DOI] [PubMed] [Google Scholar]
- Hagen A. J., Hatton T. A., Wang D. I. Protein refolding in reversed micelles. Biotechnol Bioeng. 1990 Apr 25;35(10):955–965. doi: 10.1002/bit.260351002. [DOI] [PubMed] [Google Scholar]
- Hagen A. J., Hatton T. A., Wang D. I. Protein refolding in reversed micelles: Interactions of the protein with micelle components. Biotechnol Bioeng. 1990 Apr 25;35(10):966–975. doi: 10.1002/bit.260351003. [DOI] [PubMed] [Google Scholar]
- Han D., Rhee J. S. Characteristics of lipase-catalyzed hydrolysis of olive oil in AOT-isooctane reversed micelles. Biotechnol Bioeng. 1986 Aug;28(8):1250–1255. doi: 10.1002/bit.260280817. [DOI] [PubMed] [Google Scholar]
- Hanley A. B., Furniss C. S., Kwiatkowska C. A., Mackie A. R. The manipulation of DNA with restriction enzymes in low water systems. Biochim Biophys Acta. 1991 May 24;1074(1):40–44. doi: 10.1016/0304-4165(91)90036-g. [DOI] [PubMed] [Google Scholar]
- Hilhorst R., Spruijt R., Laane C., Veeger C. Rules for the regulation of enzyme activity in reserved micelles as illustrated by the conversion of apolar steroids by 20 beta-hydroxysteroid dehydrogenase. Eur J Biochem. 1984 Nov 2;144(3):459–466. doi: 10.1111/j.1432-1033.1984.tb08488.x. [DOI] [PubMed] [Google Scholar]
- Hilhorst R., Verhaert R. M., Visser A. J. Characterization of protein-containing reversed micelles. Biochem Soc Trans. 1991 Aug;19(3):666–670. doi: 10.1042/bst0190666. [DOI] [PubMed] [Google Scholar]
- Kabanov A. V., Klyachko N. L., Nametkin S. N., Merker S., Zaroza A. V., Bunik V. I., Ivanov M. V., Levashov A. V. Engineering of functional supramacromolecular complexes of proteins (enzymes) using reversed micelles as matrix microreactors. Protein Eng. 1991 Dec;4(8):1009–1017. doi: 10.1093/protein/4.8.1009. [DOI] [PubMed] [Google Scholar]
- Katiyar S. S., Kumar A., Kumar A. The phenomenon of super activity in dihydrofolate reductase entrapped inside reverse micelles in apolar solvents. Biochem Int. 1989 Sep;19(3):547–552. [PubMed] [Google Scholar]
- Khmelnitsky Y. L., Neverova I. N., Polyakov V. I., Grinberg VYa, Levashov A. V., Martinek K. Kinetic theory of enzymatic reactions in reversed micellar systems. Application of the pseudophase approach for partitioning substrates. Eur J Biochem. 1990 May 31;190(1):155–159. doi: 10.1111/j.1432-1033.1990.tb15559.x. [DOI] [PubMed] [Google Scholar]
- Kliachko N. L., Levashov A. V., Martinek K. Kataliz fermentami, vkliuchennymi v obrashchennye mitselly poverkhnostno-aktivnykh veshchestv v organicheskikh rastvoriteliakh. Peroksidaza v sisteme aérozol' OT-voda-oktan. Mol Biol (Mosk) 1984 Jul-Aug;18(4):1019–1031. [PubMed] [Google Scholar]
- Klyachko N. L., Levashov A. V., Pshezhetsky A. V., Bogdanova N. G., Berezin I. V., Martinek K. Catalysis by enzymes entrapped into hydrated surfactant aggregates having lamellar or cylindrical (hexagonal) or ball-shaped (cubic) structure in organic solvents. Eur J Biochem. 1986 Nov 17;161(1):149–154. doi: 10.1111/j.1432-1033.1986.tb10135.x. [DOI] [PubMed] [Google Scholar]
- Kumar A., Kumar A., Katiyar S. S. Activity and kinetic characteristics of glutathione reductase in vitro in reverse micellar waterpool. Biochim Biophys Acta. 1989 Jun 13;996(1-2):1–6. doi: 10.1016/0167-4838(89)90085-x. [DOI] [PubMed] [Google Scholar]
- Kurganov B. I., Shkarina T. N., Malakhova E. A., Davydov D. R., Chebotareva N. A. Kinetics of soybean lipoxygenase reaction in hydrated reversed micelles. Biochimie. 1989 Apr;71(4):573–578. doi: 10.1016/0300-9084(89)90189-2. [DOI] [PubMed] [Google Scholar]
- Kurganov B. I., Tsetlin L. G., Malakhova E. A., Chebotareva N. A., Lankin V. Z., Glebova G. D., Berezovsky V. M., Levashov A. V., Martinek K. A novel approach to study of action of water-insoluble inhibitors of enzymic reactions. J Biochem Biophys Methods. 1985 Aug;11(2-3):177–184. doi: 10.1016/0165-022x(85)90053-3. [DOI] [PubMed] [Google Scholar]
- Larsson K. M., Adlercreutz P., Mattiasson B. Activity and stability of horse-liver alcohol dehydrogenase in sodium dioctylsulfosuccinate/cyclohexane reverse micelles. Eur J Biochem. 1987 Jul 1;166(1):157–161. doi: 10.1111/j.1432-1033.1987.tb13496.x. [DOI] [PubMed] [Google Scholar]
- Leser M. E., Wei G., Luisi P. L., Maestro M. Application of reverse micelles for the extraction of proteins. Biochem Biophys Res Commun. 1986 Mar 13;135(2):629–635. doi: 10.1016/0006-291x(86)90039-2. [DOI] [PubMed] [Google Scholar]
- Levashov A. V., Klyachko N. L., Bogdanova N. G., Martinek K. Fixation of a highly reactive form of alpha-chymotrypsin by micellar matrix. FEBS Lett. 1990 Jul 30;268(1):238–240. doi: 10.1016/0014-5793(90)81017-i. [DOI] [PubMed] [Google Scholar]
- Luisi P. L., Giomini M., Pileni M. P., Robinson B. H. Reverse micelles as hosts for proteins and small molecules. Biochim Biophys Acta. 1988 Feb 24;947(1):209–246. doi: 10.1016/0304-4157(88)90025-1. [DOI] [PubMed] [Google Scholar]
- Luisi P. L., Henninger F., Joppich M. Solubilization and spectroscopic properties of alpha-chymotrypsin in cyclohexane. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1384–1389. doi: 10.1016/0006-291x(77)90595-2. [DOI] [PubMed] [Google Scholar]
- Luisi P. L., Magid L. J. Solubilization of enzymes and nucleic acids in hydrocarbon micellar solutions. CRC Crit Rev Biochem. 1986;20(4):409–474. doi: 10.3109/10409238609081999. [DOI] [PubMed] [Google Scholar]
- Malakhova E. A., Kurganov B. I., Levashov A. V., Berezin I. V., Martinek K. Novyi podkhod k izucheniiu fermentativnykh reaktsii s uchastiem nerastvorimykh v vode substratov. Pankreaticheskaia lipaza, vkliuchennaia v obrashchennye mitselly poverkhnostno-aktivnogo veshchestva v organicheskom rastvoritele. Dokl Akad Nauk SSSR. 1983;270(2):474–477. [PubMed] [Google Scholar]
- Mao Q., Walde P., Luisi P. L. Kinetic behaviour of alpha-chymotrypsin in reverse micelles. A stopped-flow study. Eur J Biochem. 1992 Aug 15;208(1):165–170. doi: 10.1111/j.1432-1033.1992.tb17170.x. [DOI] [PubMed] [Google Scholar]
- Mao Q., Walde P. Substrate effects on the enzymatic activity of alpha-chymotrypsin in reverse micelles. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1105–1112. doi: 10.1016/0006-291x(91)91006-x. [DOI] [PubMed] [Google Scholar]
- Martinek K., Kliachko N. L., Levashov A. V., Berezin I. V. Mitselliarnaia énzimologiia. Kataliticheskaia aktivnost' peroksidazy v kolloidnom rastvore vody v organicheskom rastvoritele. Dokl Akad Nauk SSSR. 1983;269(2):491–493. [PubMed] [Google Scholar]
- Martinek K., Kliachko N. L., Levashov A. V., Berezin I. V. Mitselliarnaia énzimologiia. Kataliticheskaia aktivnost' peroksidazy v kolloidnom rastvore vody v organicheskom rastvoritele. Dokl Akad Nauk SSSR. 1983;269(2):491–493. [PubMed] [Google Scholar]
- Martinek K., Klyachko N. L., Kabanov A. V., Khmelnitsky YuL, Levashov A. V. The second E.C. Slater lecture. Micellar enzymology: its relation to membranology. Biochim Biophys Acta. 1989 Jun 6;981(2):161–172. doi: 10.1016/0005-2736(89)90024-2. [DOI] [PubMed] [Google Scholar]
- Martinek K., Levashov A. V., Khmelnitsky Y. L., Klyachko N. L., Berezin I. V. Colloidal solution of water in organic solvents: a microheterogeneous medium for enzymatic reactions. Science. 1982 Nov 26;218(4575):889–891. doi: 10.1126/science.6753152. [DOI] [PubMed] [Google Scholar]
- Martinek K., Levashov A. V., Klyachko N., Khmelnitski Y. L., Berezin I. V. Micellar enzymology. Eur J Biochem. 1986 Mar 17;155(3):453–468. doi: 10.1111/j.1432-1033.1986.tb09512.x. [DOI] [PubMed] [Google Scholar]
- Martinek K. Micellar enzymology: potentialities in fundamental and applied areas. Biochem Int. 1989 May;18(5):871–893. [PubMed] [Google Scholar]
- Marzola P., Forte C., Pinzino C., Veracini C. A. Activity and conformational changes of alpha-chymotrypsin in reverse micelles studied by spin labeling. FEBS Lett. 1991 Sep 2;289(1):29–32. doi: 10.1016/0014-5793(91)80901-e. [DOI] [PubMed] [Google Scholar]
- Nicot C., Vacher M., Denoroy L., Kahn P. C., Waks M. Limited proteolysis of myelin basic protein in a system mimetic of the myelin interlamellar aqueous space. J Neurochem. 1993 Apr;60(4):1283–1291. doi: 10.1111/j.1471-4159.1993.tb03288.x. [DOI] [PubMed] [Google Scholar]
- Nicot C., Vacher M., Vincent M., Gallay J., Waks M. Membrane proteins in reverse micelles: myelin basic protein in a membrane-mimetic environment. Biochemistry. 1985 Nov 19;24(24):7024–7032. doi: 10.1021/bi00345a041. [DOI] [PubMed] [Google Scholar]
- OGURA Y. Catalase activity at high concentration of hydrogen peroxide. Arch Biochem Biophys. 1955 Aug;57(2):288–300. doi: 10.1016/0003-9861(55)90291-5. [DOI] [PubMed] [Google Scholar]
- Oldfield C. Evaluation of steady-state kinetic parameters for enzymes solubilized in water-in-oil microemulsion systems. Biochem J. 1990 Nov 15;272(1):15–22. doi: 10.1042/bj2720015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield C., Freedman R. B. Kinetics of bilirubin oxidation catalysed by bilirubin oxidase in a water-in-oil microemulsion system. Eur J Biochem. 1989 Aug 1;183(2):347–355. doi: 10.1111/j.1432-1033.1989.tb14935.x. [DOI] [PubMed] [Google Scholar]
- Peng Q. Q., Luisi P. L. The behavior of proteases in lecithin reverse micelles. Eur J Biochem. 1990 Mar 10;188(2):471–480. doi: 10.1111/j.1432-1033.1990.tb15425.x. [DOI] [PubMed] [Google Scholar]
- Perez-Gilabert M., Sanchez-Ferrer A., Garcia-Carmona F. Application of active-phase plot to the kinetic analysis of lipoxygenase in reverse micelles. Biochem J. 1992 Dec 15;288(Pt 3):1011–1015. doi: 10.1042/bj2881011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon P. H., Wells M. A. Physical studies on egg phosphatidylcholine in diethyl ether- water solutions. Biochemistry. 1974 Nov 19;13(24):4928–4936. doi: 10.1021/bi00721a008. [DOI] [PubMed] [Google Scholar]
- Sanchez Ferrer A., Santema J. S., Hilhorst R., Visser A. J. Fluorescence detection of enzymatically formed hydrogen peroxide in aqueous solution and in reversed micelles. Anal Biochem. 1990 May 15;187(1):129–132. doi: 10.1016/0003-2697(90)90429-d. [DOI] [PubMed] [Google Scholar]
- Steinmann B., Jäckle H., Luisi P. L. A comparative study of lysozyme conformation in various reverse micellar systems. Biopolymers. 1986 Jun;25(6):1133–1156. doi: 10.1002/bip.360250612. [DOI] [PubMed] [Google Scholar]
- Verhaert R. M., Hilhorst R., Vermuë M., Schaafsma T. J., Veeger C. Description of enzyme kinetics in reversed micelles. 1. Theory. Eur J Biochem. 1990 Jan 12;187(1):59–72. doi: 10.1111/j.1432-1033.1990.tb15277.x. [DOI] [PubMed] [Google Scholar]
- Verhaert R. M., Tyrakowska B., Hilhorst R., Schaafsma T. J., Veeger C. Enzyme kinetics in reversed micelles. 2. Behaviour of enoate reductase. Eur J Biochem. 1990 Jan 12;187(1):73–79. doi: 10.1111/j.1432-1033.1990.tb15278.x. [DOI] [PubMed] [Google Scholar]
- Vos K., Laane C., Visser A. J. Spectroscopy of reversed micelles. Photochem Photobiol. 1987 Jun;45(6):863–878. doi: 10.1111/j.1751-1097.1987.tb07897.x. [DOI] [PubMed] [Google Scholar]
- Vos K., Laane C., Weijers S. R., Van Hoek A., Veeger C., Visser A. J. Time-resolved fluorescence and circular dichroism of porphyrin cytochrome c and Zn-porphyrin cytochrome c incorporated in reversed micelles. Eur J Biochem. 1987 Dec 1;169(2):259–268. doi: 10.1111/j.1432-1033.1987.tb13606.x. [DOI] [PubMed] [Google Scholar]
- Vos K., Lavalette D., Visser A. J. Triplet-state kinetics of Zn-porphyrin cytochrome c in micellar media. Measurement of intermicellar exchange rates. Eur J Biochem. 1987 Dec 1;169(2):269–273. doi: 10.1111/j.1432-1033.1987.tb13607.x. [DOI] [PubMed] [Google Scholar]
- Waks M. Proteins and peptides in water-restricted environments. Proteins. 1986 Sep;1(1):4–15. doi: 10.1002/prot.340010104. [DOI] [PubMed] [Google Scholar]
- Walde P., Han D., Luisi P. L. Spectroscopic and kinetic studies of lipases solubilized in reverse micelles. Biochemistry. 1993 Apr 20;32(15):4029–4034. doi: 10.1021/bi00066a025. [DOI] [PubMed] [Google Scholar]
- Walde P., Peng Q., Fadnavis N. W., Battistel E., Luisi P. L. Structure and activity of trypsin in reverse micelles. Eur J Biochem. 1988 Apr 15;173(2):401–409. doi: 10.1111/j.1432-1033.1988.tb14013.x. [DOI] [PubMed] [Google Scholar]
- Wojcieszyn J. W., Schlegel R. A., Wu E. S., Jacobson K. A. Diffusion of injected macromolecules within the cytoplasm of living cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4407–4410. doi: 10.1073/pnas.78.7.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R., Luisi P. L. Micellar solubilization of enzymes in hydrocarbon solvents. Enzymatic activity and spectroscopic properties of ribonuclease in N-octane. Biochem Biophys Res Commun. 1979 Jul 12;89(1):209–217. doi: 10.1016/0006-291x(79)90965-3. [DOI] [PubMed] [Google Scholar]
- van Berkel-Arts A., Dekker M., van Dijk C., Grande H. J., Hagen W. R., Hilhorst R., Krüse-Wolters M., Laane C., Veeger C. Application of hydrogenase in biotechnological conversions. Biochimie. 1986 Jan;68(1):201–209. doi: 10.1016/s0300-9084(86)81084-7. [DOI] [PubMed] [Google Scholar]