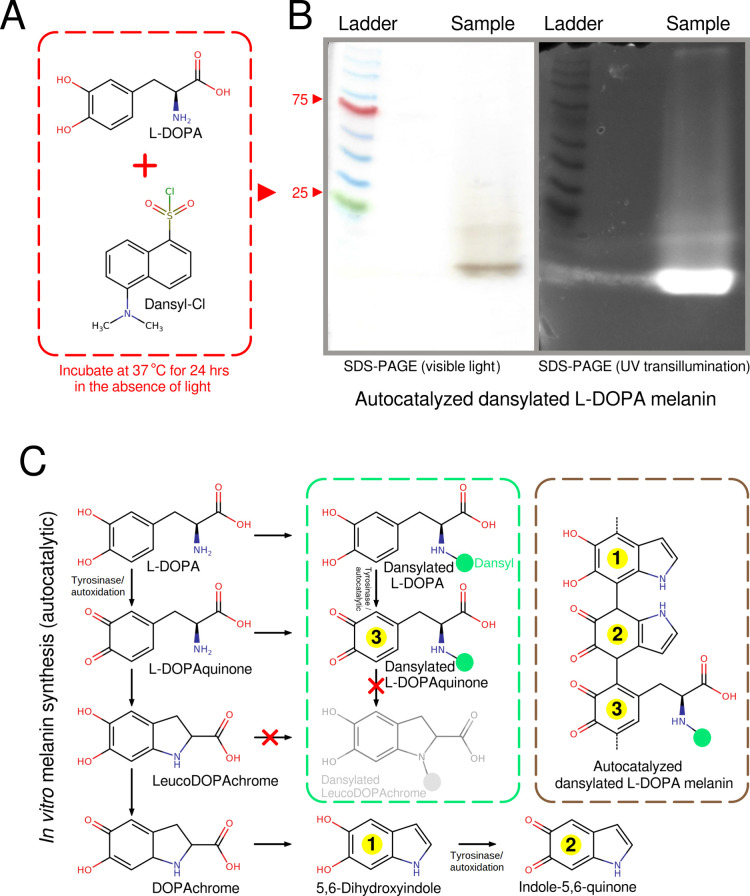
Figure 7.
In vitro formation of melanin from dansylated L-DOPA shows that it is possible for L-DOPA containing an occupied N-terminus to be incorporated into melanin. (A) L-DOPA and dansyl chloride were incubated under conditions that allowed for the dansylation of primary amines. These conditions also allowed for the in vitro autoxidation of L-DOPA into melanin. (B) An SDS PAGE gel confirms that the autoxidized L-DOPA melanin is linked to dansyl. (C) Reactions along the melanin biosynthetic pathway that can form eumelanin from L-DOPA via nonenzymatic or autodixation reactions.40 Tyrosinase can increase reaction rates41 but is not essential for the in vitro formation of eumelanin. Only L-DOPA and L-DOPAquinone possess dansylatable N-terminals (primary amines). Metabolites from leucoDOPAchrome onward only possess secondary amines and cannot be dansylated under our reaction conditions. Likewise, dansylated L-DOPAchrome, lacking a primary amine group, cannot undergo intramolecular addition (cyclization) into dansylated leucoDOPAchrome. Therefore, L-DOPA and L-DOPAquinone containing dansylated/occupied N-terminals can be incorporated into melanin.