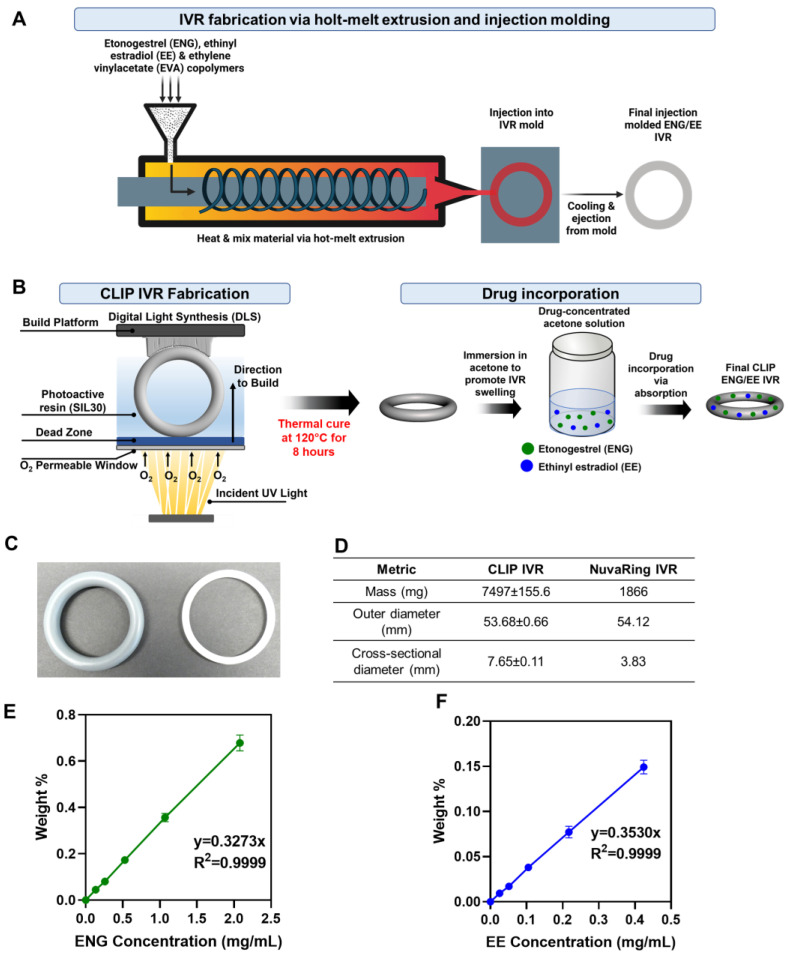
Figure 1.
Illustration of IVR manufacturing and loading equations for ENG and EE. (A) Schematic of IVR manufacturing with traditional hot-melt extrusion and injection molding. Drugs (ENG and EE) and copolymer (EVA) are poured into the hopper of the injection molding machine. Material is heated and uniformly mixed via hot-melt extrusion and injected into the final IVR mold. Once cooled, the machine ejects the final ENG/EE EVA IVR (NuvaRing). Figure made with BioRender.com. (B) IVR manufacturing with CLIP 3D printing (CLIP IVR). Projection of UV light onto photoactive resin (SIL30) promotes polymerization and solidification of the UV-cured IVR. Incorporation of oxygen via an oxygen-permeable window generates a region of uncured resin, known as the ‘dead zone’, to prevent part-attachment to the window. After the UV cure, the IVR undergoes a thermal cure to complete the fabrication process to produce the final product. ENG/EE was incorporated into the IVR via absorption as the IVR swells upon immersion in a drug-containing solution, promoting drug uptake. (C) Image of CLIP IVR (left) and NuvaRing (right). (D) Mass and dimensions of CLIP IVR (n = 3) and NuvaRing (n = 1). (E,F) Loading equations of ENG and EE in CLIP IVR, respectively, as previously developed and validated [12].