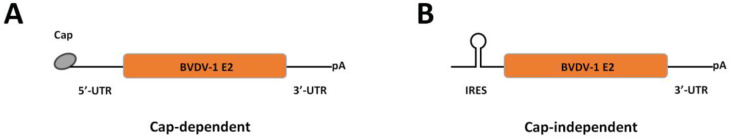
Figure 1.
Schematic diagram showing the design of the cap-dependent (A) and independent (B) mRNA vaccines. For mRNA synthesis, DNA was linearized immediately after the 3′ poly(A) via BspQI restriction digest. In vitro transcription on the linearized plasmid was carried out at 37 °C for 2 h using the T7 High-Yield RNA Transcription Kit (Vazyme TR101, Nanjing, China). Following the reaction, DNase was promptly added and incubated at 37 °C for 15 min to degrade any residual DNA template. To obtain the cap1 structure, the RNA product was purified and further modified using Vaccinia Capping Enzyme (Vazyme DD4109, Nanjing, China) and mRNA Cap 2′-O-Methyltransferase (Vazyme DD4110, Nanjing, China) according to the manufacturer’s instructions. The purity and quality of the RNAs were determined using spectrophotometric analysis (NanoDropTM One/OneC, Thermo Fisher Scientific, Shanghai, China) and agarose gel electrophoresis.