Abstract
Background
Data regarding the clinical outcome of patients with immune checkpoint inhibitor (ICI)-induced colitis are scant. We aimed to describe the 12-month clinical outcome of patients with ICI-induced colitis.
Materials and methods
This was a retrospective, European, multicentre study. Endoscopy/histology-proven ICI-induced colitis patients were enrolled. The 12-month clinical remission rate, defined as a Common Terminology Criteria for Adverse Events diarrhoea grade of 0-1, and the correlates of 12-month remission were assessed.
Results
Ninety-six patients [male:female ratio 1.5:1; median age 65 years, interquartile range (IQR) 55.5-71.5 years] were included. Lung cancer (41, 42.7%) and melanoma (30, 31.2%) were the most common cancers. ICI-related gastrointestinal symptoms occurred at a median time of 4 months (IQR 2-7 months). An inflammatory bowel disease (IBD)-like pattern was present in 74 patients (77.1%) [35 (47.3%) ulcerative colitis (UC)-like, 11 (14.9%) Crohn’s disease (CD)-like, 28 (37.8%) IBD-like unclassified], while microscopic colitis was present in 19 patients (19.8%). As a first line, systemic steroids were the most prescribed drugs (65, 67.7%). The 12-month clinical remission rate was 47.7 per 100 person-years [95% confidence interval (CI) 33.5-67.8). ICI was discontinued due to colitis in 66 patients (79.5%). A CD-like pattern was associated with remission failure (hazard ratio 3.84, 95% CI 1.16-12.69). Having histopathological signs of microscopic colitis (P = 0.049) and microscopic versus UC-/CD-like colitis (P = 0.014) were associated with a better outcome. Discontinuing the ICI was not related to the 12-month remission (P = 0.483). Four patients (3.1%) died from ICI-induced colitis.
Conclusions
Patients with IBD-like colitis may need an early and more aggressive treatment. Future studies should focus on how to improve long-term clinical outcomes.
Key words: colitis, immunotherapy, infliximab, nivolumab, pembrolizumab
Highlights
-
•
The use of ICIs is associated with an increased risk of developing different forms of colitis.
-
•
The 12-month clinical outcome of ICI-induced colitis and the factors associated with lack of remission are poorly known.
-
•
A Crohn’s disease-like pattern was associated with a worse 12-month clinical remission rate.
-
•
Having histopathological signs of microscopic colitis was associated with a better 12-month clinical remission rate.
Introduction
Immune checkpoint inhibitors (ICIs), including, among others, nivolumab, ipilimumab, atezolizumab, and pembrolizumab, are a type of immunotherapy that enhances T-lymphocyte-mediated antitumour activity through inhibition of negative co-stimulatory molecules, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and its ligand (PD-L1).1, 2, 3, 4 Advanced melanoma skin cancer, non-small-cell lung carcinoma, and kidney adenocarcinoma are the major indications, even if, in more recent years, these have further expanded.4
Despite their undoubted effectiveness in treating cancer, the use of ICI is burdened by side-effects that are mostly related to the aberrant T-cell activation. This translates into a greater risk of developing autoimmune thyroid disease, hypophysitis, dermatitis, and interstitial lung disease.5, 6, 7 The gastrointestinal tract, especially the colon, is one of the most common target sites after the endocrine glands and the skin; a form of ICI-induced colitis has been reported in roughly 20%-30% of patients taking an ICI.5,8 Apart from colitis, other gastrointestinal toxicities include acute gastritis, enteropathy, hepatitis, and pancreatitis.5
Most data about ICI-induced colitis derive from real-world studies (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103632)9, 10, 11, 12, 13, 14, 15, 16 carrying some limitations, such as a short follow-up, a small sample size, and the lack of endoscopic and histopathological data. In many cases, the presence of colitis has been presumed just because of diarrhoea, without carrying out endoscopy with histological assessment. For all these reasons, the epidemiology and the clinicopathological spectrum of ICI-induced colitis are still elusive, and data regarding treatment outcomes, the potential risk factors for a more aggressive disease, and proper management are limited. Although the American Gastroenterological Association (AGA) has drafted a clinical practice update on ICI-induced colitis,17 and guidelines on the management of ICI-related adverse events have been recently updated by authoritative scientific societies (European Society for Medical Oncology [ESMO] and American Society of Clinical Oncology [ASCO]),18,19 current treatment algorithms are not based on solid evidence, as there are no available randomised clinical trials.
Starting from these premises, the aim of this retrospective observational study was to investigate the clinical, endoscopic, and histopathological features of a series of patients with ICI-induced colitis or enterocolitis across several European centres, with at least 12 months of clinical follow-up after the first index colonoscopy. Additionally, we sought to describe potential risk factors associated with the failure to reach 12-month clinical remission.
Materials and methods
The study proponents (MVL, DGR) designed and submitted the protocol to the Clinical Commission (ClinCom) of the European Crohn’s and Colitis Organisation (ECCO) in 2020; the definitive protocol was eventually approved in 2022.
This was a multicentric, retrospective, observational study. The study was promoted through the ECCO channels, and, eventually, 20 European centres (from seven different countries, namely Italy, Turkey, Austria, Malta, Greece, Belgium, and Poland) decided to take part. A prospective study, enrolling and following up patients with ICI-induced colitis, is currently ongoing, and it is not the object of the present paper.
The study population consisted of any cancer patient who was started on any available ICI and who subsequently developed colitis, with the following inclusion criteria: age ≥18 years, ability to provide informed consent (waiving of the informed consent was applied if national regulations allowed to do so, as detailed later), undergoing at least one infusion of ICI before the onset of colitis, and having at least one colonoscopy/proctosigmoidoscopy showing macroscopic and/or histological colitis. For the primary outcome (see later), we included only patients with a follow-up of at least 12 months, unless death occurred before that time point. All patients diagnosed in each centre since 2016, and until January 2022, were included.
Demographic and clinical data
Several demographic and clinical characteristics were collected through the local electronic medical records, in particular, age, sex, tumour localisation (i.e. skin, lung, kidney, colorectal, and others) and histology, tumour stage [i.e. TNM (tumour–node–metastasis) classification], type of ICI (i.e. ipilimumab, nivolumab, pembrolizumab, atezolizumab, combination therapy, other ICI), and oncological response to ICI (i.e. complete response, partial response, disease progression). Data regarding colonoscopy, histology, inflammatory markers (i.e. C-reactive protein, faecal calprotectin), and clinical manifestations were assessed for each patient, as well as the therapy (first, second, and third line, when applicable) and outcome. All gastrointestinal symptoms were recorded; diarrhoea was graded from 1 to 5, according to the Common Terminology Criteria for Adverse Events (CTCAE).20 Additionally, data regarding the presence of comorbidities were recorded, including autoimmune disorders and the Charlson Comorbidity Index.21
Classification of colitis
Currently, a formal classification of ICI-induced colitis does not exist, and a varied nomenclature has been used in previous studies. Nonetheless, three major forms have been repeatedly reported,22,23 namely microscopic colitis (either lymphocytic or collagenous), inflammatory bowel disease (IBD)-like colitis [both ulcerative colitis (UC)-like and Crohn’s disease (CD)-like], and IBD-like unclassified. Microscopic colitis was diagnosed following the current guidelines24 for this condition; i.e. increased colonic intraepithelial lymphocytes (i.e. lymphocytic colitis) or thickening of the subepithelial collagen band (i.e. collagenous colitis), in the absence of endoscopic IBD-like inflammatory signs. UC-like and CD-like colitis were instead diagnosed when patients had clear endoscopic findings resembling UC (i.e. continuous inflammation starting from the ampulla up to the caecal valve) or CD (i.e. skip lesions, serpiginous ulcers, terminal ileum or proximal small bowel involvement), respectively, as per dedicated IBD guidelines.25,26 We labelled as ‘indeterminate colitis’ those cases that did not clearly meet the characteristics of the aforementioned forms of colitis. Finally, patients with endoscopic IBD-like forms of colitis, but also displaying histopathological features of microscopic colitis, were labelled as IBD-like. A histopathological sub-analysis, regardless of the endoscopic appearance, had also been carried out (see the details later).
Study outcomes
As a primary outcome, we have assessed the rate of ICI-induced colitis remission at 12 months (or last follow-up in case death occurred before), defined as remission of diarrhoea or colitis according to the CTCAE. Remission was defined as CTCAE diarrhoea or colitis grade of 1 or 0. The steroid-free 12-month clinical remission rate was also assessed.
The key secondary outcome was to identify potential correlates of lack of remission at 12 months, including, among others, age, sex, type of ICI, the presence of comorbidities as assessed with the Charlson Comorbidity Index, the type of colitis, the tumour stage, and others. Other secondary outcomes included the description of the epidemiological characteristics of ICI-induced colitis, the endoscopic and histological features, the death rate, and the causes of death. A histopathological sub-analysis for patients displaying intraepithelial lymphocytosis or subepithelial collagen band thickening, regardless of the endoscopic appearance, was also carried out.
Ethical and privacy considerations
Written informed consent was provided by patients or caregivers before taking part in the study, according to the approved protocol by the local ethics committee (29 July 2022; P39798/22; Fondazione IRCCS Policlinico San Matteo), extended to all the participating centres. In some countries other than Italy, written informed consent was not requested, as per local regulations, in case the patient had deceased at the time of the study initiation and because of the retrospective design. All data were reported in accordance with the STROBE recommendations for quality assurance. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Data availability statement
All relevant data generated in the research project are reported in the present paper. The raw data of the study cannot be made public due to privacy restrictions but can be shared upon reasonable request to the corresponding author.
Statistics
Data were analysed using the Stata software (release 17, StataCorp, College Station, TX). This being an observational, retrospective, study, no efficacy and safety measures were needed, neither were any mandatory study procedures. Study flow chart included one baseline evaluation at the time of ICI-induced colitis diagnosis (T0) and all the available time points (approximating to 3-month time points, i.e. 3, 6, 9, and 12 months). All data were collected into a dedicated database residing on the REDCap platform at Fondazione IRCCS Policlinico San Matteo in Pavia. The platform REDCap, which is an electronic, online tool for data collection, was used by all participants identified by a personal user ID and password. All patients were pseudo-anonymized.
A descriptive statistical analysis was carried out for clinical features, and categorical/continuous data were expressed as counts and percentage/mean and standard deviation or median and 25th-75th percentiles [interquartile range (IQR)], respectively. Missing observations were excluded for percentage calculation. The sample size was primarily based on the feasibility and on the available data in the literature. The precision of the estimate of the primary endpoint (rate of remission) was computed. We originally planned to enrol 100 (±10) cases over 1 year, expecting a 1-year remission rate of 50%-60%. Based on this hypothesis, we calculated the precision of our estimates, measured as half the 95% confidence interval (CI), and the number of covariates that can be fitted (see key secondary endpoint), considering a 1 : 10 covariates to remission numbers in a multivariable model.
The association of a series of predefined non-collinear baseline covariates and remission was assessed using multivariable logistic regression. Huber–White robust standard errors were computed to account for intracentre correlation of measures. In case of death before the 12-month assessment, the last available measure was used. A sensitivity analysis of the primary endpoint was carried out using multiple imputation of the primary endpoint for those patients who did not reach the 12-month assessment due to death or loss to follow-up. A sensitivity analysis of the primary endpoint was carried out as described in the preceding text with death considered as a failure.
Results
General demographic and clinical characteristics
Overall, data from 246 patients were inserted in the database. Of these, three patients were excluded as the diagnosis of ICI-induced colitis was questioned, four refused to take part in the study, while 143 were removed because the diagnosis was not based on endoscopy and histology but just on clinical characteristics. Hence, a total of 96 patients (57 males, male:female ratio 1.5 : 1, all white Caucasians; median age 65 years, IQR 55.5-71.5 years) were included. The demographic and general clinical characteristics of patients are reported in Table 1. A minority of patients (29, 30.2%) were aged 70 years or more, most patients were no- or sporadic drinkers (66/71, 92.9%), and most patients were past or current smokers (any type of tobacco or electronic cigarettes; 51/83, 61.4%). Only 19 patients (19.8%) had no comorbidities other than cancer. Pre-existing autoimmune disorders were present in 13 cases (13.5%), with autoimmune thyroid disease being the most common (9/13, 69.2%). The cancer characteristics, their therapy, and response to treatment are reported in Table 2. Lung cancer was the most common (41, 42.7%), followed by melanoma skin cancer (30, 31.2%); in most cases, as expected, cancers were in an advanced stage at the time of enrolment. Pembrolizumab and nivolumab were the most prescribed drugs; a combination therapy was given in 18 patients (18.7%). Concurrent chemotherapy was given to 41 patients (42.7%), while radiotherapy was given to 26 patients (27.1%). Besides ICI-induced colitis, 27 patients (28.1%) developed at least another ICI-related, immune-mediated adverse event.
Table 1.
Demographic and general clinical characteristics of the 96 included patients
| Variable | |
|---|---|
| Age, years, median (IQR) | 65 (55.5-71.5) |
| Age >70 years, n (%) | 29 (30.2) |
| Sex, male, n (%) | 57 (59.3) |
| Body mass index, median (IQR) | 23.4 (21.0-26.1) |
| Alcohol consumption, n (%)a | |
| None | 41 (57.8) |
| Sporadic | 25 (35.2) |
| Daily, <40 g | 4 (5.6) |
| Daily, >40 g | 1 (1.4) |
| Smoking, n (%)b | |
| Never smoker | 32 (38.5) |
| Past smoker | 38 (45.8) |
| Current smoker | 13 (15.7) |
| Charlson Comorbidity Index, median (IQR) | 9 (7-10) |
| No other comorbidities, n (%) | 19 (19.8) |
| Pre-existing autoimmune disorders, n (%) | 13 (13.5) |
| Autoimmune thyroid disease | 9 (69.2) |
| Sjögren syndrome | 1 (7.7) |
| Rheumatoid arthritis | 1 (7.7) |
| Other not specified | 2 (15.4) |
IQR, interquartile range.
Information available in 71/96 patients.
Information available in 83/96 patients.
Table 2.
Cancer characteristics and related therapy and response
| Tumour localisation, n (%) | |
| Lung | 41 (42.7) |
| Skin | 30 (31.2) |
| Kidney | 9 (9.4) |
| Colorectal | 2 (2.1) |
| Other | 14 (14.6) |
| Histology, n (%) | |
| Adenocarcinoma | 39 (40.6) |
| Melanoma | 35 (36.5) |
| Epithelial carcinoma | 2 (2.1) |
| Squamous cell carcinoma | 12 (12.5) |
| Other | 8 (8.3) |
| TNM classification, n (%) | |
| Tumour | |
| Tx | 15 (15.6) |
| T1 | 2 (2.1) |
| T2 | 14 (14.4) |
| T3 | 27 (28.3) |
| T4 | 27 (28.3) |
| Unknown | 11 (11.3) |
| Nodes | |
| Nx | 10 (10.4) |
| N0 | 16 (16.7) |
| N1 | 26 (27.1) |
| N2 | 17 (17.7) |
| N3 | 15 (15.6) |
| Unknown | 12 (12.5) |
| Metastasis | |
| Mx | 4 (4.2) |
| M0 | 30 (31.3) |
| M1 | 59 (61.4) |
| Unknown | 3 (3.1) |
| Cancer therapy, n (%) | |
| Concomitant chemotherapy | 41 (42.71) |
| Radiotherapy | 26 (27.1) |
| Immunotherapy | |
| Ipilimumab | 9 (9.3) |
| Pembrolizumab | 43 (44.8) |
| Nivolumab | 21 (21.9) |
| Atezolizumab | 3 (3.1) |
| Combination therapy | 18 (18.7) |
| Nivolumab + ipilimumab | 13 (72.5) |
| Atezolizumab + bevacizumab | 1 (5.5) |
| Pembrolizumab + pemetrexed | 1 (5.5) |
| Pembrolizumab+ axitinib | 1 (5.5) |
| Pembrolizumab + olaparib | 1 (5.5) |
| Pembrolizumab + favezelimab | 1 (5.5) |
| Unknown | 2 (2.1) |
| Cancer response to therapy at follow-up, n (%) | |
| Stable disease | 25 (26.0) |
| Complete remission | 12 (12.5) |
| Partial remission | 40 (41.7) |
| Progression of disease | 18 (18.8) |
| Unknown | 1 (1.0) |
| Other immune-related adverse events, n (%) | |
| None | 69 (71.9) |
| Autoimmune thyroid disease | 6 (6.2) |
| Hypophysitis | 1 (1.1) |
| Autoimmune hepatitis | 1 (1.1) |
| Adrenalitis | 1 (1.1) |
| Dermatitis | 2 (2.1) |
| Interstitial lung disease | 3 (3.1) |
| Other | 13 (13.5) |
Other tumour localisation includes bladder, cavum nasi, liver, uterus, eye, jaw, mouth, pleura, prostate. Other histology includes hepatocellular carcinoma, renal cell carcinoma, sarcoma, urothelial carcinoma, clear renal cell carcinoma, small cell carcinoma.
TNM, tumour–node–metastasis.
Clinical, laboratory, endoscopic, and histopathological characteristics of colitis
Table 3 reports the clinical, laboratory, endoscopic, and histological characteristics of ICI-induced colitis. From a clinical point of view, ICI-related symptoms occurred at a median time of 4 months (IQR 2-7 months) since ICI initiation. At the onset of ICI-induced colitis, diarrhoea was present in all cases (96, 100%), being moderate-to-severe in most cases. Other symptoms/signs included rectal bleeding (6, 6.2%) and abdominal pain (5, 5.2%). Anaemia was present in 16 patients (16.7%), while the median C-reactive protein was 2.8 mg/dl (IQR 0.6-10.8 mg/dl), and the median faecal calprotectin was 765 μg/g (IQR 300-1870 μg/g). At onset, only two patients had a normal faecal calprotectin (i.e. ≤50 μg/g).
Table 3.
Clinical, laboratory, endoscopic, and histological characteristics of colitis at the time of diagnosis
| Clinical features, n (%) | |
| Diarrhoea | 96 (100) |
| Abdominal pain | 5 (5.2) |
| Rectal bleeding | 6 (6.2) |
| CTCAE classification for diarrhoea, n (%) | |
| Grade 1 | 11 (11.5) |
| Grade 2 | 27 (28.1) |
| Grade 3 | 50 (52.1) |
| Grade 4 | 6 (6.3) |
| Unknown | 2 (2.0) |
| CTCAE classification for colitis, n (%) | |
| Grade 1 | 23 (23.9) |
| Grade 2 | 40 (41.7) |
| Grade 3 | 23 (23.9) |
| Grade 4 | 5 (5.2) |
| Unknown | 5 (5.2) |
| Laboratory values, median (IQR) | |
| Haemoglobin | 12.7 (11.1-14) g/dl |
| Platelets | 219 (221-369) ∗ 109/ml |
| White blood cells | 8.8 (6.4-12.6) ∗ 109/ml |
| Neutrophils | 5.59 (3.8 8.48) ∗ 109/ml |
| Lymphocytes | 1.6 (1.1-2.3) ∗109/ml |
| Monocytes | 0.75 (0.5-0.9) ∗109/ml |
| C-reactive protein | 2.8 (0.6-10.8) mg/dl |
| Faecal calprotectin | 765 (300-1870) mcg/g |
| Endoscopy, n (%) | |
| IBD-like | 74 (77.1) |
| CD-like | 11 (14.9) |
| UC-like | 35 (47.3) |
| IBD-unclassified | 28 (37.8) |
| Microscopic colitis (macroscopically normal) | 19 (19.8) |
| Indeterminate colitis | 3 (3.1) |
| Endoscopy in IBD-like patients, n (%) | |
| Terminal ileum only | 1 (1.3) |
| Terminal ileum + colon (any site) | 4 (5.4) |
| Right colon | 2 (2.7) |
| Left colon | 35 (47.3) |
| Entire colon | 18 (24.3) |
| Rectum only | 9 (12.2) |
| Unknown | 5 (6.7) |
| Histology, n (%) | |
| Intraepithelial lymphocytosis | 31 (32.3) |
| Collagenous band thickening | 14 (14.6) |
| Crypt abscesses/cryptitis | 51 (53.1) |
| Apoptosis | 20 (20.8) |
| Gland architectural abnormalities | 25 (26.0) |
| Prominent eosinophilia | 12 (12.5) |
| Basal lymphoplasmacytosis | 0 (0) |
| Granulomas | 0 (0) |
| Other | 15 (15.6) |
Other histological features include chronic inflammatory infiltrate, increased cellularity, histiocyte aggregates.
CD, Crohn’s disease; CTCAE, Common Terminology Criteria for Adverse Events; IBD, inflammatory bowel disease; IQR, interquartile range; UC, ulcerative colitis.
Regarding endoscopy, ICI-induced colitis had an IBD-like pattern in 74 patients (77.1%), of whom 35 (47.3%) had a UC-like pattern, 11 (14.9%) had a CD-like pattern, and 28 (37.8%) had an IBD-like unclassified pattern. The terminal ileum showed macroscopic inflammation in 5/74 cases (6.7%). Overall, the left colon, with or without other colonic segments, was the most affected part of the bowel (53/74, 71.6%).
A diagnosis of microscopic colitis was made on endoscopic and histological grounds in 19 patients (19.8%), of whom 8 (9.6%) had lymphocytic colitis and 11 (13.2%), collagenous colitis. Three remaining patients (3.1%) showed no endoscopic signs of inflammation and had mild lymphocytosis, a slight thickening of the subepithelial collagenous band, and also signs of active colitis (cryptitis); for these reasons, these patients were labelled as having an indeterminate form of colitis.
Overall, histologically, basal lymphoplasmocytosis, granulomas, and Paneth cell metaplasia, which are suggestive for a primary IBD, were absent in all cases. Besides a chronic inflammatory infiltrate (56, 75.7%), the most common histopathological findings of IBD-like colitis were eosinophilic infiltration (12, 12.5%), gland architectural abnormalities (25, 26.0%), apoptotic bodies (20, 20.8%), and crypt abscesses/cryptitis (51, 27.1%). A thickening of the subepithelial collagen band and intraepithelial lymphocytosis, which are characteristics of microscopic colitis, were also noticed in 3/74 (4.1%) and in 23/74 (31.1%) patients with IBD-like colitis, respectively. These latter patients were still categorized as having either a UC- or a CD-like colitis as per the endoscopic appearance.
Finally, in only two cases, both having an IBD-like colitis, a concomitant atrophic enteropathy was reported (moderate-to-severe villous atrophy), with negative coeliac disease serology and no concomitant common variable immunodeficiency.
Colitis treatment and study outcomes
Table 4 reports the first-, second-, and third-line therapies for treating ICI-induced colitis. As a first line, systemic steroids (prednisone or others) were by far the most prescribed drugs (65, 67.7%), followed by oral budesonide (12, 12.5%). Of note, unspecified anti-diarrhoeal drugs were also prescribed in 14 cases (14.6%), having a colitis grade 1 or 2 in most cases, while biologic therapy was never prescribed as a first-line agent. Five patients with grade 1 colitis and one patient with grade 2 colitis did not receive any treatment at first. In 4/5 of these grade 1 colitis patients and in the patient with grade 2 colitis, ICI was discontinued and later recommenced. As a second-line (44 patients, 45.8%), infliximab was the most prescribed drug (15, 15.6%), followed by systemic steroids, vedolizumab (two cases), and others. Total colectomy was carried out in one patient with CTCAE grade 4 who was urgently admitted to hospital for septic shock and colonic perforation. Only a minority of patients (10, 10.4%) needed a third-line therapy. Overall, only in 10 cases (10.4%) was a topical therapy given through enemas (steroids or 5-amonosalicylates). ICI-induced colitis led to hospitalisation in 37 cases (38.5%), and steroids were given intravenously in 33 cases (89.2%). Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103632, cumulatively reports therapies stratified for the different grades of colitis at onset.
Table 4.
First-, second-, and third-line therapies for treating colitis
| First-line, n (%)a | |
| None | 6 (6.3) |
| Systemic steroids | 65 (67.7) |
| Oral budesonide | 12 (12.5) |
| Anti-diarrhoeal drugs | 14 (14.6) |
| 5-aminosalicylates | 5 (5.2) |
| Second-line, n (%)a | |
| None | 50 (52.0) |
| Infliximab | 15 (15.6) |
| Vedolizumab | 2 (2.0) |
| Systemic steroids | 11 (11.5) |
| Oral budesonide | 4 (4.2) |
| Metronidazole | 11 (11.5) |
| Total colectomy | 1 (1.0) |
| Third-line, n (%)a | |
| None | 66 (68.7) |
| Systemic steroids | 3 (3.1) |
| Oral budesonide | 3 (3.1) |
| Vedolizumab | 2 (2.1) |
| Tacrolimus | 2 (2.1) |
In some cases, more than one therapy was given to the same patient.
Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103632, shows the variation of C-reactive protein and faecal calprotectin at different time points (T0-3-6-9-12 months).
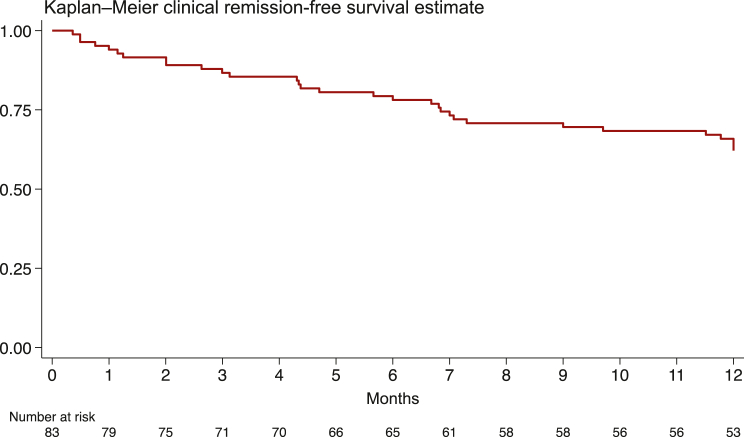
In 83/96 (86.4%) patients, the primary endpoint was available (i.e. 12-month remission or last follow-up if death occurred before 12 months); 13 patients were excluded because the 12-month outcome was either unknown, uncertain, or could not be retrieved. Clinical colitis remission was reached in 31/83 (37.4%), as defined by the diarrhoea CTCAE, with a rate of 47.7 per 100 person-years (upper and lower 95% CI bounds 33.5-67.8). At 12 months, most patients (55, 66.3%) were still on a therapy for treating ICI-induced colitis. Within the observation period, ICI was discontinued due to colitis in 66 patients (79.5%) and due to other reasons (i.e. cancer progression, other adverse events) in 8 patients (9.6%). The Kaplan–Meier clinical remission-free survival estimate is shown in Figure 1. Twelve-month steroid-free clinical remission was instead reached in 13/83 (15.7%), with a rate of 20.0 per 100 person-years (upper and lower 95% CI bounds 11.6-34.4). The Kaplan–Meier steroid-free clinical remission survival estimate is shown in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103632.
Figure 1.
Kaplan–Meier clinical remission-free survival estimate (at 12 months).
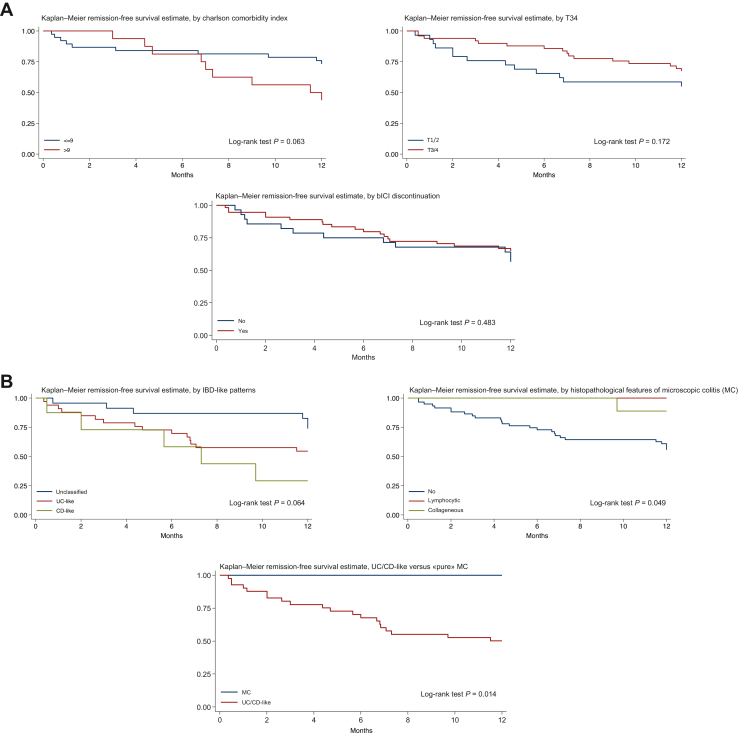
Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.103632, shows the covariate analyses for predictors of lack of clinical remission at 12 months. Pembrolizumab was borderline significantly associated with a greater likelihood of reaching remission [hazard ratio (HR) 0.35, 95% CI 0.12-1.01, P = 0.051], while a CD-like pattern to a greater likelihood of failing remission (HR 3.84, 95% CI 1.16-12.69, P = 0.03). Figure 2A shows the Kaplan–Meier remission-free estimate by Charlson Comorbidity Index (≤9 versus >9), by tumour stage (1-2 versus 3-4), and by discontinuation of ICI (yes versus no), while Figure 2B shows the Kaplan–Meier remission-free estimate by IBD-like patterns (UC-like versus CD-like versus IBD-like unclassified), by histopathological microscopic colitis pattern (lymphocytic versus collagenous versus non-microscopic colitis features), and by UC-/CD-like pattern versus microscopic colitis. Notably, a significantly more favourable outcome (i.e. reaching remission at 12 months) was noticed in patients having histopathological signs of microscopic colitis (regardless of the endoscopic appearance) versus those who not having (P = 0.049), and in patients having microscopic colitis (i.e. with no signs of endoscopic inflammation) versus UC-/CD-like pattern (P = 0.014). Patients with an IBD-like unclassified pattern had a borderline significant (P = 0.064) better outcome than those with a UC- or CD-like pattern. Discontinuing the ICI was not related to the 12-month clinical remission (P = 0.483). Particularly, patients who had resumed the ICI at 12 months (29 patients, 34.9%) compared to those who had not (37 patients, 44.6%) did not display a different outcome (P = 0.395).
Figure 2.
Kaplan-Meier remission-free curves according to different variables of interest. (A) Kaplan–Meier remission-free estimate by Charlson Comorbidity Index (≤9 versus >9), by tumour stage (1-2 versus 3-4), and by discontinuation of immune checkpoint inhibitor (yes versus no). (B) Kaplan–Meier remission-free estimate by IBD-like patterns (UC-like versus CD-like versus IBD-like unclassified), by histopathological microscopic colitis pattern (lymphocytic versus collagenous versus non-microscopic colitis features), and by UC-/CD-like pattern versus ‘pure’ microscopic colitis. CD, Crohn’s disease; IBD, inflammatory bowel disease; UC, ulcerative colitis.
Overall, 31 patients (32.3%) died at 12-month follow-up, with a rate of 19.1 per 100 person-years (lower and upper 95% CI bounds 11.1-32.8). The Kaplan–Meier survival estimate is shown in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103632. Among these 31 patients, 22 (71.0%) died of cancer progression, 4 (12.9%) of ICI-induced colitis, and 5 (16.1%) of other causes. Among the ‘other causes’, three septic shocks were reported, potentially related to ICI-induced colitis or its treatment.
Discussion
We have herein reported the 12-month clinical outcomes of a multicentric, well-characterised, cohort of endoscopy- and histopathology-confirmed ICI-induced colitis patients enrolled across seven European countries, by clearly classifying them into IBD-like colitis or microscopic colitis. Overall, these forms of colitis have a low mortality rate, but 12-month clinical remission is reached in only about one-third of patients (37.4%), with a high need for long-term therapy, and a very low steroid-free remission rate. Also, some important predictors of remission emerged. All these results are novel and have a valuable clinical significance.
Regarding the general clinical aspects, as expected, considering the main ICI indications, the most common cancer types were lung cancer and melanoma skin cancer. Additionally, most patients were either active or past smokers, and a high prevalence of multimorbidity was noticed. Overall, the prevalence of other immune-mediated ICI-related adverse events was comparable to that reported in the literature.5, 6, 7 Similarly, the median time of onset of diarrhoea (4 months) is in line with other previously published papers.27 Most patients had a moderate-to-severe diarrhoea, according to the CTCAE (≥2; 86.5%), which seems to be higher compared to the available literature, and this may reflect the specific setting of enrolment, i.e. a tertiary referral level. Faecal calprotectin was markedly raised in almost all cases at colitis onset, with a median of more than 700 μg/g; hence, similarly to IBD,28 this marker may be considered useful for discriminating colonic causes of diarrhoea while taking an ICI, and thus the need for a colonoscopy. Surprisingly, despite the severity of colitis, anaemia was rather rare, and C-reactive protein just slightly increased. This corroborates the previous hypothesis that the severity of colitis and the overall clinical picture may be dissociated.29 In other terms, a severe form of colitis may be present even in mildly symptomatic patients.
One of the most important results in terms of ICI-induced colitis presentation is the deep characterisation of the endoscopic and pathological features, which in many previous studies have been overlooked or not reported in all patients. In roughly 80% of the cases, our patients displayed endoscopic IBD-like features, more commonly UC-like, with the left colon being the most common colonic portion involved. On histopathology, granulomas, basal lymphoplasmocytosis, and Paneth cell metaplasia were always absent. Other pathological aspects, such as chronic inflammation, cryptitis, and gland architectural abnormalities, are certainly unspecific, although it is worth noting quite a high prevalence of epithelial apoptotic bodies and lamina propria eosinophilic infiltration. All these pathological features have been systematically reported only in a very small series of eight patients treated with anti-PD-1 agents, in which the authors postulated that ICI-induced colitis may resemble graft-versus-host disease-related colitis or other forms of drug-induced colitis.30
Regarding therapy, steroids have been the most used medications, while biological drugs, namely infliximab and vedolizumab, have been rarely used. This finding raises two important points. Firstly, the management of ICI-induced colitis may be suboptimal across different European countries. In fact, according to the ESMO/ASCO guidelines and AGA recommendations,17, 18, 19 despite a lack of solid evidence, infliximab and vedolizumab,31 and even other biologics or small molecules, should be offered in case of severe colitis.17, 18, 19 Instead, although promising,32 none of our patients underwent faecal microbiota transplantation. Secondly, systemic steroids might be over-used, and, surprisingly, other drugs which have no evidence of effectiveness in this setting, such as anti-diarrhoeal drugs or mesalamine, were also prescribed. The sole, temporary discontinuation of ICI was instead recommended only in a minority of cases. Systemic steroid-sparing strategies are strongly needed to allow patients to stay on ICI therapy, which, in many cases, represents the last cancer therapeutic chance. Considering that the 12-month clinical remission was reached in less than half patients, and steroid-free remission in an even smaller proportion of patients, this clearly points at the need for large, prospective, randomised clinical trials assessing the effectiveness of biologics or other treatments. Standard management protocols and therapeutic endpoints are also eagerly awaited as they might improve important outcomes. Additionally, our data raise some concerns about the potential lack of confidence among oncologists in the use of biological therapies, such as infliximab, vedolizumab, and others, with the need to consult a gastroenterologist for guidance, as well as highlight the possible concerns among gastroenterologists about the safety of biologic treatments in oncological patients. For sure, these patients would be better managed by a multidisciplinary team composed of oncologists and gastroenterologists who are expert in the management of ICI-induced colitis.
Regarding potential factors affecting clinical response, IBD-like colitis, especially CD-like, had a worse prognosis. These patients may therefore benefit from a timely and more aggressive treatment. A novel, favourable, prognostic factor emerged, i.e. the presence of intraepithelial lymphocytosis and significant subepithelial collagen thickening. These features may help stratify the risk of ongoing colonic inflammation. Finally, the discontinuation of ICI did not seem to be related to the 12-month clinical outcome. Although this datum should be cautiously interpreted, it could have important clinical implications. Further prospective studies are needed to better identify those patients who may not discontinue the ICI or who may safely restart an ICI after its discontinuation.
Indeed, our study has some limitations that should be mentioned. Firstly, given the retrospective nature, we could not retrieve more detailed data at intermediate time points. Compared to other settings, we may have included here more severe patients, thus justifying the rather low rate of 12-month clinical remission. Also, a more ambitious 12-month goal could not be assessed, i.e. mucosal healing, as a follow-up endoscopy was often missing; this could be particularly important in those patients failing to reach a clinical remission within that time frame. A detailed sub-analysis for the type of ICI and type of colitis treatment could not be made either, due to the relatively small sample size, and the endoscopy and pathology reports were not centrally reviewed. Further, we could not retrieve precise data about steroid doses, tapering modalities, and biologic drugs regimens. Nevertheless, all our patients had a clear endoscopic and histopathological diagnosis, and this is certainly the major strength of this research since most of the previously reported series are only based on symptoms. By classifying this condition into microscopic colitis versus IBD-like, relevant findings emerged. A larger prospective study for confirming our data is currently ongoing.
Conclusion
To conclude, the long-term management of ICI-induced colitis remains a challenge in clinical practice. Despite being considered and treated with protocols like that of ‘true’ IBD, our data and the available literature highlight that ICI-induced colitis may be a distinct entity with an elusive pathogenesis and uncertain natural history. The prognostic factors found in our study, although need to be confirmed on a larger scale, are key to optimising the management of these patients and provide a solid background for designing future research and guidelines. In future trials, steroid-free clinical remission should be considered as a major endpoint.
Acknowledgements
We thank the ECCO ClinCom for providing guidance in the writing of the protocol.
Funding
The study was funded by Fondazione IRCCS Policlinico San Matteo—Progetto di ricerca corrente 2022; this was a competitive grant (no grant number). The European Crohn’s and Colitis Organisation (ECCO) Clinical Commission (ClinCom) reviewed, approved the study protocol, and disseminated it to the ECCO members, but did not act as a sponsor or promoter.
Disclosure
MVL has served as speaker for AbbVie, Takeda, Ferring and has received research grants from Takeda and Janssen. EVS has served as speaker for AbbVie, Agave, AGPharma, Alfasigma, Aurora Pharma, CaDiGroup, Celltrion, Dr Falk, EG Stada Group, Fenix Pharma, Fresenius Kabi, Galapagos, Janssen, JB Pharmaceuticals, Innovamedica/Adacyte, Malesci, Mayoly Biohealth, Omega Pharma, Pfizer, Reckitt Benckiser, Sandoz, SILA, Sofar, Takeda, Tillots, and Unifarco; has served as consultant for AbbVie, Agave, Alfasigma, Biogen, Bristol-Myers Squibb, Celltrion, Diadema Farmaceutici, Dr. Falk, Fenix Pharma, Fresenius Kabi, Janssen, JB Pharmaceuticals, Merck & Co, Nestlè, Reckitt Benckiser, Regeneron, Sanofi, SILA, Sofar, Synformulas GmbH, Takeda, and Unifarco; he received research support from Pfizer, Reckitt Benckiser, SILA, Sofar, Unifarco, and Zeta Farmaceutici. LB has received lecture fees, consultancy fees, or advisory board honoraria from AbbVie, Aliveda, Janssen, Noos, Pfizer, Pharmanutra, Takeda, and Zambon. DP got speaker’s fee/advisory board from Janssen, Pfizer, Galapagos, Takeda, MSD, AbbVie, and Biogen. RS has received speaker’s fees from AbbVie. MC has served as advisory board member and received fees for invited lectures from Biogen, Janssen, Galapagos, Takeda, Pfizer, Ferring, Fresenius, Celltrion, and Alnylam. PE received lecture fees and travel grants from Ferring and Takeda. IK has served as advisory board member and received fees for invited lectures from AbbVie, MSD, Janssen, Takeda, Pfizer, Ferring, Vianex, Viatris, and Faran. AA received consulting/advisory board fees from AbbVie, Alfa-Sigma, Amgen, Astra Zeneca, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb,Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Giuliani, Janssen,Lionhealth, MSD, Nestlé, Pfizer, Protagonist Therapeutics, Roche, Sanofi, Samsung Bioepis, Sandoz, Takeda, Tillots Pharma Speaker’s fees from AbbVie, AG Pharma, Amgen, Biogen, Bristol-Myers Squibb, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, Lionealth, MSD, Novartis, Pfizer, Roche, SamsungBioepis, Sandoz, Takeda, Teva Pharmaceuticals. AB has received lecture or consultancy fees from AbbVie, Aengus, Boston Scientific, Bristol-Myers Squibb, Falk, Genericon, Gilead, Janssen, MSD, Olympus, Pfizer, Takeda, Vifor. All other authors have declared no potential conflicts of interest.
Contributor Information
A. Di Sabatino, Email: a.disabatino@smatteo.pv.it.
European Consortium for the study of immune checkpoint inhibitor-induced colitis:
C. Mengoli, N. Aronico, F. Lepore, G. Broglio, S. Merli, G. Natalello, E. Alimenti, D. Scalvini, S. Muscarella, F. Agustoni, A. Pagani, S. Chiellino, S. Corallo, V. Musella, R. Cannizzaro, M. Vecchi, F. Caprioli, R. Gabbiadini, A. Dal Buono, A. Premoli, L.D. Locati, A. Buda, A. Contaldo, A. Schiepatti, F. Biagi, D. Morano, M. Cucè, A. Kotsakis, and G. De Lisi
Supplementary data
References
- 1.Bagchi S., Yuan R., Engleman E.G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 2.Granier C., De Guillebon E., Blanc C., et al. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open. 2017;2 doi: 10.1136/esmoopen-2017-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiravand Y., Khodadadi F., Kashani S.M.A., et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. 2022;29:3044–3060. doi: 10.3390/curroncol29050247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin-Acevedo J.A., Kimbrough E.O., Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14:45. doi: 10.1186/s13045-021-01056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martins F., Sofiya L., Sykiotis G.P., et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 6.Johnson D.B., Nebhan C.A., Moslehi J.J., Balko J.M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254–267. doi: 10.1038/s41571-022-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan R.J., Weber J.S. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov. 2022;21:495–508. doi: 10.1038/s41573-021-00259-5. [DOI] [PubMed] [Google Scholar]
- 8.Wang D.Y., Ye F., Zhao S., Johnson D.B. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: a systematic review and meta-analysis. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1344805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander J.L., Ibraheim H., Sheth B., et al. Clinical outcomes of patients with corticosteroid refractory immune checkpoint inhibitor-induced enterocolitis treated with infliximab. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson D.H., Zobniw C.M., Trinh V.A., et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer. 2018;6:103. doi: 10.1186/s40425-018-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Abu-Sbeih H., Mao E., et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6:37. doi: 10.1186/s40425-018-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou F., Faleck D., Thomas A., et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu-Sbeih H., Ali F.S., Wang X., et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2019;7:93. doi: 10.1186/s40425-019-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mooradian M.J., Wang D.Y., Coromilas A., et al. Mucosal inflammation predicts response to systemic steroids in immune checkpoint inhibitor colitis. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes M.S., Molina G.E., Chen S.T., et al. Budesonide treatment for microscopic colitis from immune checkpoint inhibitors. J Immunother Cancer. 2019;7:292. doi: 10.1186/s40425-019-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geukes Foppen M.H., Rozeman E.A., van Wilpe S., et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3 doi: 10.1136/esmoopen-2017-000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dougan M., Wang Y., Rubio-Tapia A., Lim J.K. AGA Clinical Practice Update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis: expert review. Gastroenterology. 2021;160:1384–1393. doi: 10.1053/j.gastro.2020.08.063. [DOI] [PubMed] [Google Scholar]
- 18.Haanen J, Obeid M, Spain L, et al., ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1217-1238. [DOI] [PubMed]
- 19.Schneider B.J., Naidoo J., Santomasso B.D., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO Guideline Update. J Clin Oncol. 2021;39:4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute Cancer Therapy Evaluation Program. https://ctep.cancer.gov/protocolDevelopment/adverse_effects.htm Available at.
- 21.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Som A., Mandaliya R., Alsaadi D., et al. Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J Clin Cases. 2019;7:405–418. doi: 10.12998/wjcc.v7.i4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung V.T.F., Gupta T., Olsson-Brown A., et al. Immune checkpoint inhibitor-related colitis assessment and prognosis: can IBD scoring point the way? Br J Cancer. 2020;123:207–215. doi: 10.1038/s41416-020-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miehlke S., Guagnozzi D., Zabana Y., et al. European guidelines on microscopic colitis: United European Gastroenterology and European Microscopic Colitis Group statements and recommendations. United European Gastroenterol J. 2021;9:13–37. doi: 10.1177/2050640620951905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raine T., Bonovas S., Burisch J., et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16:2–17. doi: 10.1093/ecco-jcc/jjab178. [DOI] [PubMed] [Google Scholar]
- 26.Torres J., Bonovas S., Doherty G., et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. J Crohns Colitis. 2020;14:4–22. doi: 10.1093/ecco-jcc/jjz180. [DOI] [PubMed] [Google Scholar]
- 27.Postow M.A. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015;35:76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- 28.Walsham N.E., Sherwood R.A. Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol. 2016;9:21–29. doi: 10.2147/CEG.S51902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Abu-Sbeih H., Mao E., et al. Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis. 2018;24:1695–1705. doi: 10.1093/ibd/izy104. [DOI] [PubMed] [Google Scholar]
- 30.Chen J.H., Pezhouh M.K., Lauwers G.Y., Masia R. Histopathologic features of colitis due to immunotherapy with anti-PD-1 antibodies. Am J Surg Pathol. 2017;41:643–654. doi: 10.1097/PAS.0000000000000829. [DOI] [PubMed] [Google Scholar]
- 31.d’Apolito M., Spagnuolo R., Siciliano M.A., et al. Autoimmune colitis and neutropenia in adjuvant anti-PD-1 therapy for malignant melanoma: efficacy of vedolizumab, a case report. Ther Adv Chronic Dis. 2022;13 doi: 10.1177/20406223211063024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Wiesnoski D.H., Helmink B.A., et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24:1804–1808. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data generated in the research project are reported in the present paper. The raw data of the study cannot be made public due to privacy restrictions but can be shared upon reasonable request to the corresponding author.