Abstract
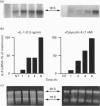
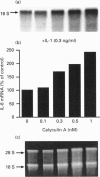
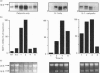
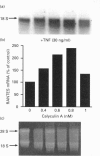
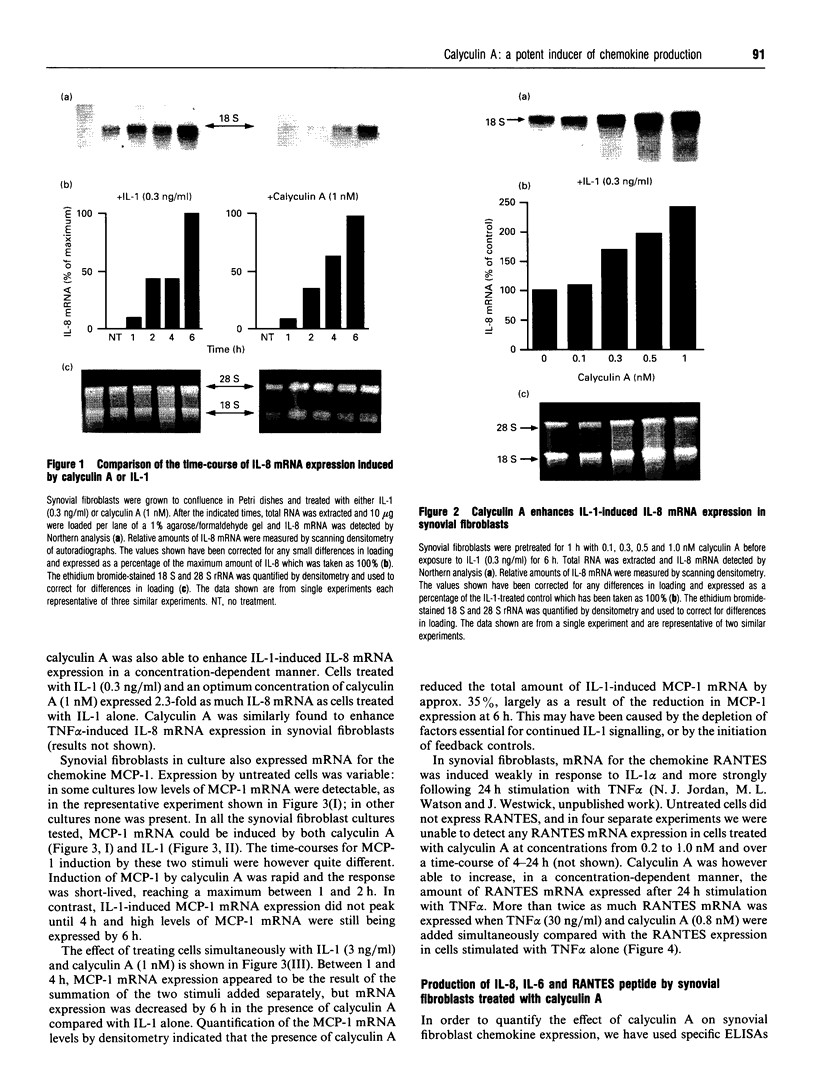
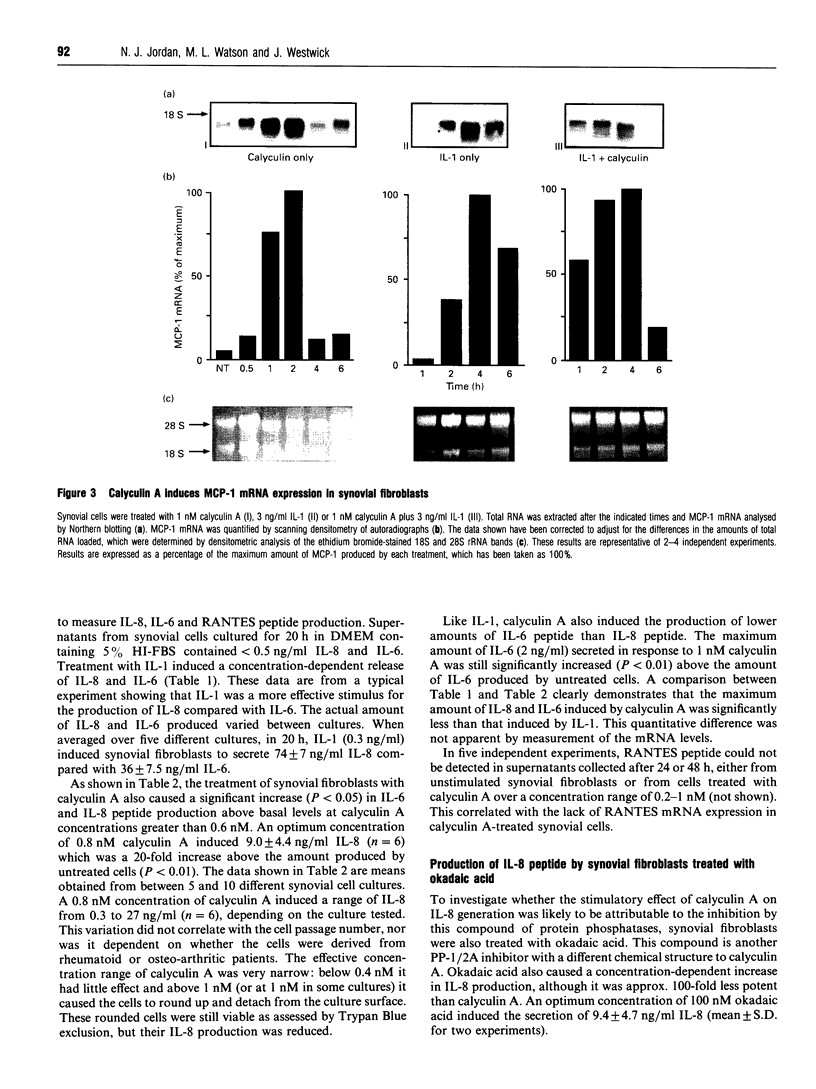
Cultured human synovial fibroblasts express mRNA for the chemotactic cytokines (chemokines) interleukin-8 (IL-8), monocyte chemotactic protein 1 (MCP-1) and regulated upon activation normal T-cell expressed and presumably secreted (RANTES), when stimulated with IL-1 or tumour necrosis factor alpha (TNF alpha). Calyculin A, a potent type 1/2A protein serine/threonine phosphatase inhibitor, was used to examine the role of protein phosphatases in the regulation of chemokine gene expression. Calyculin A (1 nM) mimicked IL-1 by inducing IL-8 and MCP-1 mRNA expression in synovial cells. IL-8 mRNA was induced over a similar time period (1-6 h) in response to IL-1 or calyculin A, whereas MCP-1 mRNA was induced more rapidly (1-2 h) by calyculin A than by IL-1 (4-6 h). Expression of RANTES mRNA occurred in response to TNF alpha, but could not be induced by stimulation with calyculin A alone. These results suggest that inhibition of protein phosphatase type 1/2A may have a differential role in the regulation of the expression of each of the chemokine genes. Synovial fibroblasts also secreted IL-8 and IL-6 peptide when stimulated with either IL-1/TNF alpha or calyculin A. The amount of IL-8 and IL-6 peptide produced in response to calyculin A was significantly increased above that produced by untreated synovial cells, though it was much less than the amount induced by IL-1 or TNF alpha. Calyculin A also acted synergistically with IL-1 or TNF alpha to cause a 2-fold potentiation of IL-1- or TNF alpha-induced IL-8 mRNA and peptide and RANTES mRNA expression. These results suggest that although inhibition of a protein phosphatase may be able to regulate the magnitude of IL-1-induced chemokine gene expression, the IL-1 signal transduction pathway involves components in addition to phosphatase inhibition, possibly including the activation of a protein kinase, the action of which may be opposed by a protein phosphatase inhibited by calyculin A.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessi D. R., Smythe C., Keyse S. M. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993 Jul;8(7):2015–2020. [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Finco T. S., Nantermet P. V., Baldwin A. S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993 Jun;13(6):3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird T. A., Sleath P. R., deRoos P. C., Dower S. K., Virca G. D. Interleukin-1 represents a new modality for the activation of extracellular signal-regulated kinases/microtubule-associated protein-2 kinases. J Biol Chem. 1991 Nov 25;266(33):22661–22670. [PubMed] [Google Scholar]
- Bohjanen P. R., Petryniak B., June C. H., Thompson C. B., Lindsten T. An inducible cytoplasmic factor (AU-B) binds selectively to AUUUA multimers in the 3' untranslated region of lymphokine mRNA. Mol Cell Biol. 1991 Jun;11(6):3288–3295. doi: 10.1128/mcb.11.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard P. A., Golds E. E. Cytokine-induced expression of mRNAs for chemotactic factors in human synovial cells and fibroblasts. J Cell Physiol. 1993 Feb;154(2):433–441. doi: 10.1002/jcp.1041540227. [DOI] [PubMed] [Google Scholar]
- Casillas A. M., Amaral K., Chegini-Farahani S., Nel A. E. Okadaic acid activates p42 mitogen-activated protein kinase (MAP kinase; ERK-2) in B-lymphocytes but inhibits rather than augments cellular proliferation: contrast with phorbol 12-myristate 13-acetate. Biochem J. 1993 Mar 1;290(Pt 2):545–550. doi: 10.1042/bj2900545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Brewis N. D., Hughes V., Mann D. J. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 1990 Aug 1;268(2):355–359. doi: 10.1016/0014-5793(90)81285-v. [DOI] [PubMed] [Google Scholar]
- Davis P. D., Elliott L. H., Harris W., Hill C. H., Hurst S. A., Keech E., Kumar M. K., Lawton G., Nixon J. S., Wilkinson S. E. Inhibitors of protein kinase C. 2. Substituted bisindolylmaleimides with improved potency and selectivity. J Med Chem. 1992 Mar 20;35(6):994–1001. doi: 10.1021/jm00084a004. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco D., Kunkel S. L., Strieter R. M., Basha M., Zurier R. B. Interleukin-1 induced gene expression of neutrophil activating protein (interleukin-8) and monocyte chemotactic peptide in human synovial cells. Biochem Biophys Res Commun. 1991 Jan 31;174(2):411–416. doi: 10.1016/0006-291x(91)91431-b. [DOI] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Gorospe M., Kumar S., Baglioni C. Tumor necrosis factor increases stability of interleukin-1 mRNA by activating protein kinase C. J Biol Chem. 1993 Mar 25;268(9):6214–6220. [PubMed] [Google Scholar]
- Guy G. R., Cairns J., Ng S. B., Tan Y. H. Inactivation of a redox-sensitive protein phosphatase during the early events of tumor necrosis factor/interleukin-1 signal transduction. J Biol Chem. 1993 Jan 25;268(3):2141–2148. [PubMed] [Google Scholar]
- Guy G. R., Cao X., Chua S. P., Tan Y. H. Okadaic acid mimics multiple changes in early protein phosphorylation and gene expression induced by tumor necrosis factor or interleukin-1. J Biol Chem. 1992 Jan 25;267(3):1846–1852. [PubMed] [Google Scholar]
- Guy G. R., Chua S. P., Wong N. S., Ng S. B., Tan Y. H. Interleukin 1 and tumor necrosis factor activate common multiple protein kinases in human fibroblasts. J Biol Chem. 1991 Aug 5;266(22):14343–14352. [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Harlow L. A., Johnson B., Evanoff H. L., Haines G. K., Burdick M. D., Pope R. M., Strieter R. M. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992 Sep;90(3):772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Martin M., Resch K. Interleukin 1: more than a mediator between leukocytes. Trends Pharmacol Sci. 1988 May;9(5):171–177. doi: 10.1016/0165-6147(88)90033-8. [DOI] [PubMed] [Google Scholar]
- Matsusaka T., Fujikawa K., Nishio Y., Mukaida N., Matsushima K., Kishimoto T., Akira S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L. T., Helfgott D. C., Sehgal P. B. Anti-beta-interferon antibodies inhibit the increased expression of HLA-B7 mRNA in tumor necrosis factor-treated human fibroblasts: structural studies of the beta 2 interferon involved. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8957–8961. doi: 10.1073/pnas.83.23.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S. D., Qin S., Guy G. R., Tan Y. H. Differential induction of nuclear NF-kappa B by protein phosphatase inhibitors in primary and transformed human cells. Requirement for both oxidation and phosphorylation in nuclear translocation. J Biol Chem. 1993 Dec 15;268(35):26805–26812. [PubMed] [Google Scholar]
- Mukaida N., Mahe Y., Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990 Dec 5;265(34):21128–21133. [PubMed] [Google Scholar]
- Nelson P. J., Kim H. T., Manning W. C., Goralski T. J., Krensky A. M. Genomic organization and transcriptional regulation of the RANTES chemokine gene. J Immunol. 1993 Sep 1;151(5):2601–2612. [PubMed] [Google Scholar]
- Peichl P., Ceska M., Effenberger F., Haberhauer G., Broell H., Lindley I. J. Presence of NAP-1/IL-8 in synovial fluids indicates a possible pathogenic role in rheumatoid arthritis. Scand J Immunol. 1991 Sep;34(3):333–339. doi: 10.1111/j.1365-3083.1991.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Rathanaswami P., Hachicha M., Sadick M., Schall T. J., McColl S. R. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993 Mar 15;268(8):5834–5839. [PubMed] [Google Scholar]
- Rot A., Krieger M., Brunner T., Bischoff S. C., Schall T. J., Dahinden C. A. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992 Dec 1;176(6):1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Toy K. J., Goeddel D. V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990 Oct 18;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Jongstra J., Dyer B. J., Jorgensen J., Clayberger C., Davis M. M., Krensky A. M. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988 Aug 1;141(3):1018–1025. [PubMed] [Google Scholar]
- Shyy Y. J., Li Y. S., Kolattukudy P. E. Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem Biophys Res Commun. 1990 Jun 15;169(2):346–351. doi: 10.1016/0006-291x(90)90338-n. [DOI] [PubMed] [Google Scholar]
- Stephens J. M., Carter B. Z., Pekala P. H., Malter J. S. Tumor necrosis factor alpha-induced glucose transporter (GLUT-1) mRNA stabilization in 3T3-L1 preadipocytes. Regulation by the adenosine-uridine binding factor. J Biol Chem. 1992 Apr 25;267(12):8336–8341. [PubMed] [Google Scholar]
- Strieter R. M., Phan S. H., Showell H. J., Remick D. G., Lynch J. P., Genord M., Raiford C., Eskandari M., Marks R. M., Kunkel S. L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989 Jun 25;264(18):10621–10626. [PubMed] [Google Scholar]
- Sung S. J., Walters J. A. Stimulation of interleukin-1 alpha and interleukin-1 beta production in human monocytes by protein phosphatase 1 and 2A inhibitors. J Biol Chem. 1993 Mar 15;268(8):5802–5809. [PubMed] [Google Scholar]
- Taktak Y. S., Selkirk S., Bristow A. F., Carpenter A., Ball C., Rafferty B., Poole S. Assay of pyrogens by interleukin-6 release from monocytic cell lines. J Pharm Pharmacol. 1991 Aug;43(8):578–582. doi: 10.1111/j.2042-7158.1991.tb03540.x. [DOI] [PubMed] [Google Scholar]
- Takuma T., Ichida T., Okumura K., Kanazawa M. Protein phosphatase inhibitor calyculin A induces hyperphosphorylation of cytokeratins and inhibits amylase exocytosis in the rat parotid acini. FEBS Lett. 1993 May 24;323(1-2):145–150. doi: 10.1016/0014-5793(93)81467-e. [DOI] [PubMed] [Google Scholar]
- Vietor I., Schwenger P., Li W., Schlessinger J., Vilcek J. Tumor necrosis factor-induced activation and increased tyrosine phosphorylation of mitogen-activated protein (MAP) kinase in human fibroblasts. J Biol Chem. 1993 Sep 5;268(25):18994–18999. [PubMed] [Google Scholar]
- Villiger P. M., Terkeltaub R., Lotz M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J Immunol. 1992 Jul 15;149(2):722–727. [PubMed] [Google Scholar]
- Watson M. L., Westwick J., Fincham N. J., Camp R. D. Elevation of PMN cytosolic free calcium and locomotion stimulated by novel peptides from IL-1-treated human synovial cell cultures. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1154–1160. doi: 10.1016/s0006-291x(88)81261-0. [DOI] [PubMed] [Google Scholar]
- Wright S. C., Zheng H., Zhong J., Torti F. M., Larrick J. W. Role of protein phosphorylation in TNF-induced apoptosis: phosphatase inhibitors synergize with TNF to activate DNA fragmentation in normal as well as TNF-resistant U937 variants. J Cell Biochem. 1993 Nov;53(3):222–233. doi: 10.1002/jcb.240530307. [DOI] [PubMed] [Google Scholar]
- Xia H. Z., Kannapell C. C., Fu S. M., Sung S. S. Differential regulation of human B-lymphocyte tumor necrosis factor-alpha (TNF-alpha) and lymphotoxin (TNF-beta) production by protein phosphatase 1 and 2A inhibitor. Blood. 1993 Nov 1;82(9):2806–2812. [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Robinson E. A., Tanaka S., Appella E., Kuratsu J., Leonard E. J. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989 Apr 1;169(4):1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Moore S. K., Appella E., Lerman M. I., Leonard E. J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989 Feb 27;244(2):487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]