FIG A1.
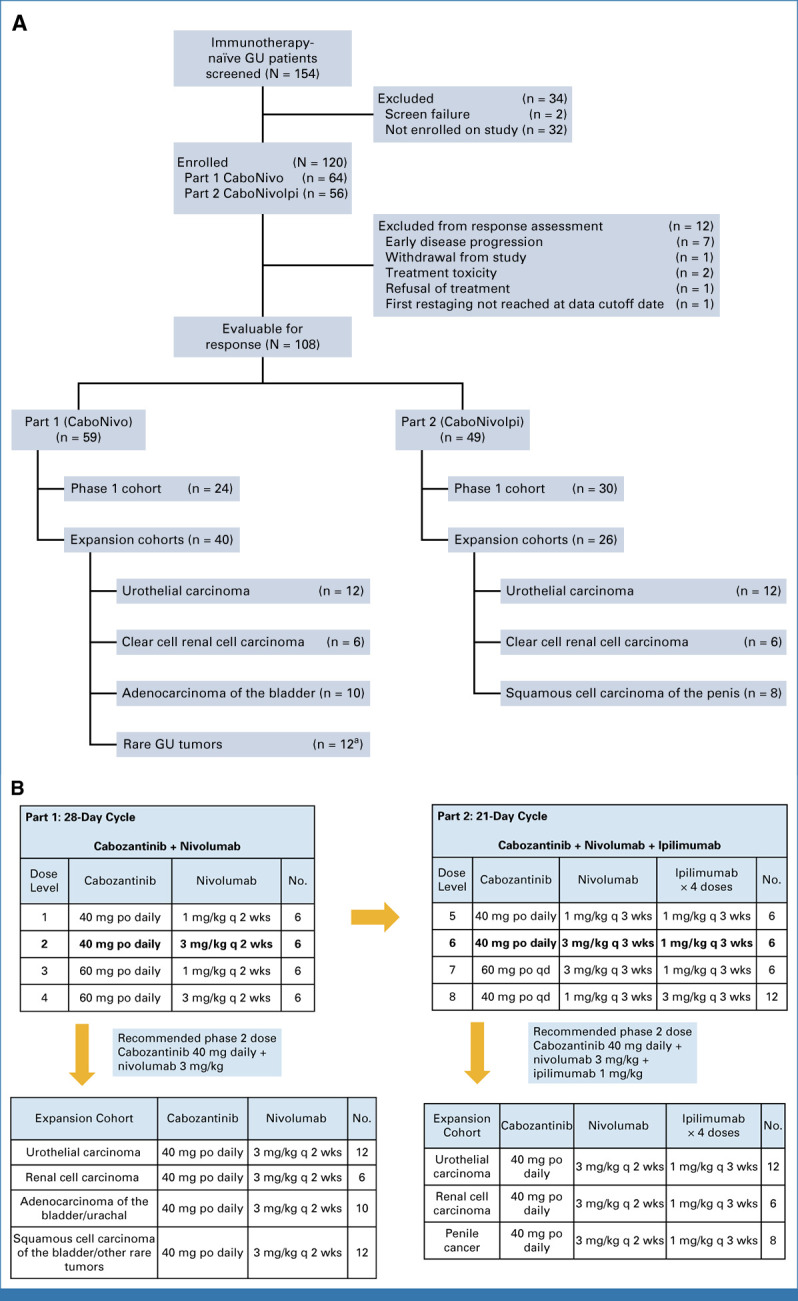
Study design and dose-escalation schematic for CaboNivo and CaboNivoIpi and respective expansion cohorts. (A) CONSORT diagram of patient flow. (B) Dose levels and corresponding doses for cabozantinib plus nivolumab (Part 1—CaboNivo) and cabozantinib, nivolumab, and ipilimumab (Part 2—CaboNivoIpi). Lower panels list the tumor histologies accrued within the expansion cohorts for CaboNivo and CaboNivoIpi. aHistologies enrolled into rare genitourinary tumors cohort included squamous cell carcinoma of the bladder (n = 5), collecting duct carcinoma of the kidney (n = 1), sarcomatoid RCC (n = 1), chromophobe RCC (n = 1), small cell carcinoma of the bladder (n = 1), papillary RCC (n = 1), bladder adenocarcinoma (n = 1), and sarcomatoid bladder (n = 1). GU, genitourinary; po, orally; q 2 wks, once every 2 weeks; q 3 wks, once every 3 weeks; qd, once daily.
