FIG A2.
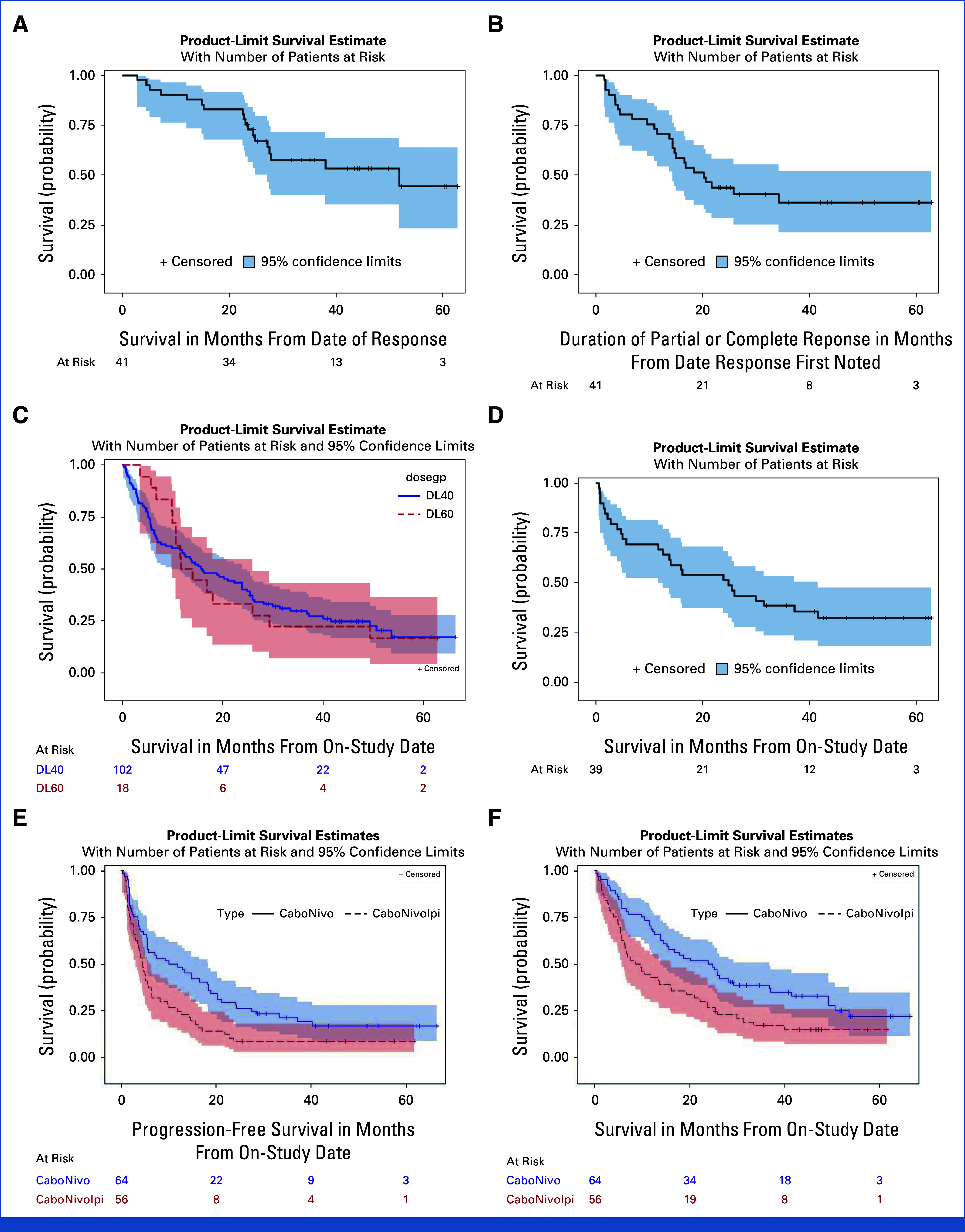
Exploratory Kaplan-Meier estimate of PFS, OS, and DoR for subgroups. (A) OS for responders (CR + PR) (n = 41) (median OS: 51.8 months [95% CI, 27.1 months to not estimable]; 6 month OS: 92.7% [95% CI, 79.0% to 97.6%]; 12 month OS: 90.2% [95% CI, 76.1% to 96.2%]; 24 month OS: 72.8% [95% CI, 56.3% to 86.6%]). (B) Median DoR for responders (median duration of PR, CR: 20.2 months [95% CI, 14.4 months to not estimable]; 6 month DOR: 80.5% [95% CI, 64.8% to 89.7%]; 12 month DOR: 70.7% [95% CI, 54.3% to 82.2%]; 24 month DOR: 43.9% [95% CI, 28.6% to 58.2%]). (C) OS (N = 120) by cabozantinib dose 40 mg (median OS: 15.8 months [95% CI, 9.9 months to 23.9 months]; 6 month OS: 68.6% [95% CI, 58.7% to 76.7%]; 12 month OS: 57.8% [95% CI, 47.7% to 66.7%]; 24 month OS: 40.2% [95% CI, 30.7% to 49.5%]) and 60 mg (N = 120) (median OS 12.9 months [95% CI, 10.0 months to 26.0 months]; 88.9% [95% CI, 62.4% to 97.1%]; 50.0% [95% CI, 25.9% to 70.1%]; 33.3% [95% CI, 13.7% to 54.5%]). (D) OS for patients with UC (n = 39) (median OS: 24.9 months [95% CI, 11.8 months to 41.6 months]; 6 month OS: 69.2% [95% CI, 52.2% to 81.2%]; 12 month OS: 66.7% [95% CI, 49.6% to 79.1%]; 24 month OS: 51.3% [95% CI, 34.8% to 65.5%]). (E) PFS in patients treated with CaboNivo and CaboNivoIpi (median PFS: CaboNivo: 11.0 months [95% CI, 5.5 months to 18.4 months] CaboNivoIpi: 4.5 months [95% CI, 3.1 months to 5.8 months] P = 0.0069). (F) OS in patients treated with CaboNivo and CaboNivoIpi (median OS CaboNivo: 24.4 months [95% CI, 14.0 months to 36.8 months]; median OS CaboNivoIpi: 9.3 months [95% CI, 5.8 months to 15.9 months] P = 0.0072). CR, complete response; DoR, duration of response; OS, overall survival; PFS, progression-free survival; PR, partial response; UC, urothelial carcinoma.
