Key Clinical Message
Stone heart syndrome has a complex interaction with digoxin toxicity, where, theoretically, the administration of intravenous calcium can worsen a patient's condition. Research on this subject is conflicting, so it is imperative to approach it cautiously.
Keywords: anti‐digoxin Fab, calcium, digoxin toxicity, hyperkalemia
1. INTRODUCTION
Ischemic myocardial contracture, better known as stone heart syndrome, is a rare yet often fatal condition that occurs during cardiac bypass surgeries, specifically aortic valve replacement and coronary artery bypass grafting (CABG). 1 Usually, this condition presents with myocardium hypertrophy and fibrosis but has been seen with other congenital abnormalities such as ventricular septum defects (VSD) and patent arterial duct (PDA). 1 In such cases, a stone heart was evident soon after the initiation of procedures as the left ventricle entered a “fixed state of hypercontraction”. 1 Although the mechanism is not fully understood, this paper aims to examine the correlation between digoxin toxicity and the stone heart phenomenon.
Digoxin, a pharmaceutical drug under the cardiac glycoside class, has long been utilized to treat congestive heart failure and dysrhythmia. More recently, digoxin has fallen out of favor in deference to medications such as beta blockers and ACE inhibitors. 2 Essentially, this is due to digoxin's narrow therapeutic index, multiple drug interactions, and high risk for toxicity. 2 Digoxin toxicity and its clinical treatment have been controversially linked to causing stone heart syndrome. 3 , 4 Digoxin inhibits the sodium‐potassium ATPase pump, causing potassium to become trapped extracellularly, leading to hyperkalemia. Intravenous calcium gluconate is commonly used as a first‐agent medication for hyperkalemia presenting with ECG changes to antagonize potassium's cardiac effect. 5 However, there was mixed evidence that the interaction of intravenous calcium with digoxin toxicity could cause a “stone heart.” The idea was that adding calcium, when there was already an excess within the myocytes, led to uncontrolled calcium‐troponin C binding, further depleting ATP and forming rigor bonds. 4
The interplay between digoxin toxicity and stone heart syndrome and their pharmacological effects on cardiac physiology is crucial for clinical decision‐making and improving patient outcomes. We present a detailed case to demonstrate the actual implications of this relationship and its inherent challenges in practice. It helps in bridging the theory of digoxin toxicity and stone heart syndrome with a subtle approach to treatment in the inpatient setting.
2. CASE HISTORY
A 78‐year‐old female patient with a past medical history including 5 year history of permanent atrial fibrillation (previously on Xarelto, currently off anticoagulation), hypertensive heart disease, mild intermittent asthma, and type 2 diabetes with an A1c of 6.1 (on metformin) presented to her cardiologist's office for routine follow‐up. Home medications included spironolactone, digoxin, empagliflozin, furosemide, metformin, metoprolol succinate, and diltiazem. Vital signs revealed a blood pressure of 76/50 mmHg and a heart rate of 108. It was documented that she did not report any symptoms at that time. Laboratory tests revealed elevated potassium levels at 6 mmol/L and a creatinine level of 1.55 mg/dL, which was raised from baseline, suggesting acute kidney injury. The patient was referred to the Emergency Department, where she mentioned to medical staff that she had multiple episodes of vomiting with minimal food and water intake for a few days prior to the presentation. Laboratories that resulted in the ED were as follows: WBC 9.1 cells/mcL, Na 131 mmol/L, K 6.1 mmol/L, Cr 1.77 mg/dL, and digoxin 1.1 ng/mL. The details are shown in Table 1. She was administered calcium gluconate 1 g IV, inhaled albuterol, intravenous insulin, and 500 mL normal saline bolus in the Emergency Department. The patient's blood pressure improved, and she was admitted to the hospital for hyperkalemia secondary to her acute kidney injury and concomitant spironolactone use. Acute kidney injury was thought to be multifactorial and related to recent vomiting, decreased oral intake, and diuretic use. She was started on normal saline with repeat laboratories showing resolution in her hyperkalemia. Her home digoxin, spironolactone, diltiazem, and metoprolol succinate were continued.
TABLE 1.
Laboratory results on admission and upon escalation of care to the intensive care unit.
| Latest reference range and units | Day 1 | Day 1 | Day 1 | Day 2 | Day 2 | Day 2 | |
|---|---|---|---|---|---|---|---|
| 11:17 | 15:00 | 18:06 | 08:01 | 12:33 | 15:35 | ||
| Sodium | 135–145 mmol/L | 133 (L) | 131 (L) | 137 | 131 (L) | 137 | 131 (L) |
| Potassium | 3.6–5.2 mmol/L | 6.0!! | 6.1!! | 4.8 | 5.9 (H) | 5.9 (H) | 5.3 (H) |
| Chloride | 98–107 mmol/L | 108 (H) | 108 (H) | 113 (H) | 112 (H) | 115 (H) | 107 |
| Bicarbonate | 22–29 mmol/L | 15 (L) | 13 (L) | 10 (L) | 15 (L) | 13 (L) | 11 (L) |
| Anion gap | 7–15 | 10 | 10 | 14 | 4 (L) | 9 | 13 |
| BUN (Blood Urea Nitrogen) | 6–21 mg/dL | 27 (H) | 26 (H) | 26 (H) | 21 | 20 | 20 |
| Creatinine | 0.59–1.04 mg/dL | 1.55 (H) | 1.77 (H) | 1.47 (H) | 1.05 (H) | 1.01 | 1.07 (H) |
| Estimated GFR (eGFR) | > = 60 mL/min/BSA | 34 (L) | 29 (L) | 37 (L) | 55 (L) | 57 (L) | 53 (L) |
| Calcium, total | 8.8–10.2 mg/dL | 10.2 | 10.0 | 10.4 (H) | 9.8 | 9.9 | 9.4 |
| Glucose | mg/dL | 114 | 103 | Canceled | 105 | 131 | 214 (H) |
| Magnesium | 1.4 (L) | ||||||
| Digoxin | ng/mL | 1.1 | 1.9 (H) |
Note: Day 1 is the day of admission. Day 2 is when patient was transferred to the intensive care unit (!!: Data are critical, (L): Data are abnormally low, (H): Data are abnormally high).
The next day, the patient was found to be hyperkalemic and was treated with IV calcium gluconate 1 g, dextrose, and insulin. Spironolactone was discontinued. Normal saline was continued. An electrocardiogram (ECG) demonstrated no changes consistent with hyperkalemia. Approximately 2 h later, a rapid response was initiated due to significant bradycardia and hypotension—the patient's heart rate was 47 beats per minute, and blood pressure was 85/55 mmHg. Laboratories were ordered (Table 1, 12:33). Patient mentioned nonspecific abdominal pain at the time with no tenderness on examination. She denied any chest pain, shortness of breath, lightheadedness, dizziness, and vision changes. At this time, concerns for digoxin toxicity were strongly considered. Laboratories were repeated, and an EKG was performed (Figure 1). Patient was administered a 500 mL bolus of normal saline with little improvement in blood pressure.
FIGURE 1.
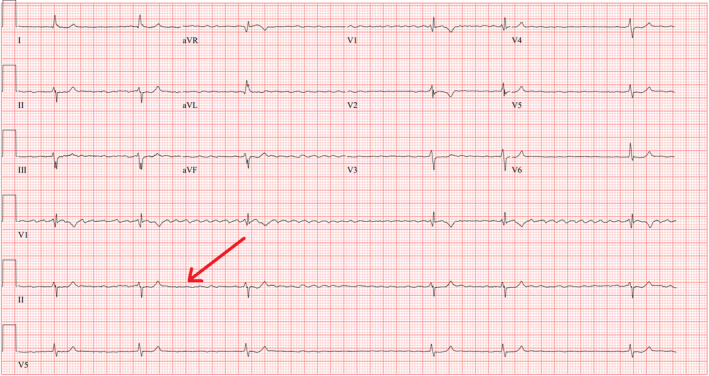
EKG was ordered at 12:23 pm, showing bradycardia (slow atrial fibrillation) at rapid response.
The digoxin level at this time was 1.9 ng/mL (therapeutic range 0.6–1.2 ng/mL), with the upper limit typically being 1.2 ng/mL. The critical care team was consulted and assessed the patient at the bedside. A bedside echocardiogram showed a decrease in contractility with ejection fraction (EF) ranging between 15% and 20%. This represented a significant decrease from her baseline EF of 49%. She was subsequently treated with 40 mg of the anti‐digoxin antigen‐binding fragment (anti‐digoxin Fab) and 2 mg of glucagon. However, no significant improvement was noted in the patient's blood pressure or heart rate, necessitating vasopressor therapy with dopamine and transfer to the intensive care unit (ICU). Two doses of Atropine 0.5 mg were administered at this time, followed by the initiation of an epinephrine infusion. The patient was also found to have a pH of 7.2 and was started on a sodium bicarbonate infusion. Poison control recommended repeat doses of anti‐digoxin Fab up to 360 mg. The patient ultimately received a total of 120 mg over 3 h and 40 min. Vasopressin infusion was initiated until improvement in acidemia. Figure 2 presents the vital signs just prior to and post initation of vasopressors and indicates the dosages of each vasopressor administered. Administration of anti‐digoxin Fab and multiple vasopressors led to return of baseline cardiac function as confirmed by repeat echocardiogram. Over the next 48 h, the patient was gradually weaned off pressor support.
FIGURE 2.
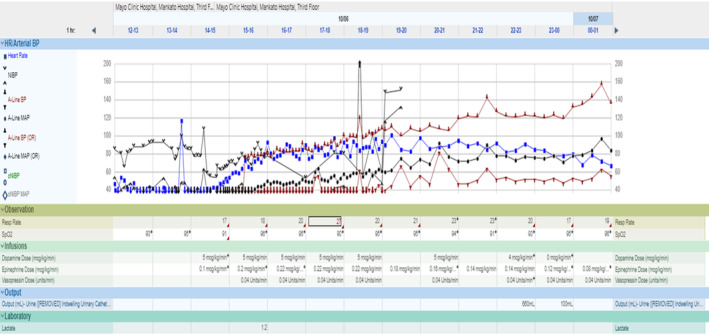
This chart shows the critical vital signs leading up to a rapid response and extending into the initial phase of the intensive care unit stay. It also details the initiation of vasopressor medications, including the specific doses administered.
3. DIFFERENTIAL DIAGNOSIS
3.1. Digoxin toxicity
Volume depletion with concomitant beta blocker, calcium blocker, and digoxin treatment.
4. OUTCOME/FOLLOW‐UP
The patient was discharged from the hospital 6 days after the presentation. Digoxin was held indefinitely. Furosemide and empagliflozin were held, given concerns that they may have contributed to volume depletion, necessitating her admission into the hospital. They were held with plans to reinitiate follow‐up cardiology appointments pending further evaluation potentially. A follow‐up echocardiography a month after discharge showed that her ejection fraction had returned to her prior baseline of 49%. She remained clinically hemodynamically stable at her 4‐week cardiology follow‐up.
5. DISCUSSION
Treatment of hyperkalemia with intravenous calcium in the setting of digoxin toxicity, in theory, can be dangerous. As mentioned, digoxin inhibits sodium‐potassium ATPase, increasing intracellular sodium concentrations and effectively inhibiting sodium‐dependent calcium transport out of the cytoplasm. 6 It also increases the activity of the sodium/calcium exchanger tasked with removing intracellular sodium in exchange for calcium. 7 This results in increased calcium concentrations within the cell and consequent increased inotropy. Intravenous calcium, once administered, would exacerbate this process. The potentiation in inotropy, if significant, can precipitate an irreversible non‐contractile state secondary to the inability of the cardiac muscle to relax during diastole due to the binding of calcium to troponin‐C. 8
Diagnosis can be challenging. It, therefore, remains imperative that other potential etiologies be ruled out. When combined, hyperkalemia, elevated digoxin levels, and an associated decrease in cardiac motility are red flags. While it can be challenging to parse out potential confounders, given the dire clinical consequences of this condition, potential cardiogenic shock and subsequent death, treatment should not be delayed.
Anti‐digoxin Fabs are a well‐known antidote for severe digoxin toxicity. Infact, it may be the only active treatment at present time for toxicity. 7 It has a variety of indications, including ventricular arrhythmias, hypotension, symptomatic bradycardia, potassium levels greater than 5 mmol/L in acute overdoses, acute ingestions exceeding 10 mg in adults or 4 mg in children, digoxin concentrations greater than 15 ng/mL or greater than 10 ng/mL 6 h after ingestion, high‐grade heart blocks. 3 Dosing is variable depending on the severity and chronicity of toxicity. Acute on chronic digoxin toxicity are similar; however, there are important differences in presentation. Acute toxicity can initially present with no symptoms with subsequent gastrointestinal issues like vomiting, nausea, and abdominal pain. Neurological symptoms, like confusion, can occur later owing to drug distribution in the central nervous system. Chronic toxicity is more difficult to diagnose given a more protracted duration of onset: in the span of days to months. Gastrointestinal symptoms are more noticeable than neurological symptoms. Both acute and chronic toxicity can include electrolyte abnormalities as well as visual disturbances. 9 In acute overdoses, 10 to 20 vials can be administered empirically for critically ill patients. In contrast, empiric dose is substantially lower in chronic toxicity and can range from 3 to 6 vials, while children slightly lower even at 1–2 vials. Dosing can also be calculated based on ingested dose or steady‐state digoxin levels and body weight. It is usually infused over 30 min but can be given more rapidly in critical cases, with effects beginning in 20 min and full efficacy in approximately 90 min. 3 If Anti‐digoxin Fab is unavailable, treatment should be geared towards mediating its consequent side effect profile. Examples include multidose‐activated charcoal, antidysrhythmics, or even cardioversion/pacing. Chronic digoxin toxicity with minimal and no changes on EKG can be managed by discontinuing the medication or decreasing the dose. 10 Activated charcoal can be used in the first 2 h of acute ingestion provided patient does not have an impaired level of consciousness. 10 Antidysrhythmics like lidocaine and magnesium sulfate can be used for ventricular arrhythmias and atropine for bradyarrhythmias. 10 If anti‐digoxin Fab is unavailable, cardiac pacing may need to be considered. 10
Anti‐digoxin Fab was administered in the setting of hyperkalemia and shock. As digoxin toxicity was in the differential, it was imperative that patient was treated immediately. Hyperkalemia can have a host of different etiologies, including but not limited to, hyperglycemia, metabolic acidosis, medications, and acute kidney injury (AKI). In our case, the patient presented with hyperkalemia, which was ascribed to her AKI as digoxin levels were within normal limits at that time. Digoxin was continued, and hyperkalemia was treated with improvement. The following day, the patient developed hyperkalemia once again; however, in this instance, they no longer had an acute kidney injury. It was, therefore, likely that digoxin was the culprit behind this repeated episode of hyperkalemia. An alternate etiology may have been related to patient's spirinolactone in the setting of kidney injury. As discussed, IV calcium was administered, and within a few hours, the patient developed cardiogenic shock. Digoxin levels were found to be elevated on repeat blood work. The patient was also on a beta blocker and a calcium channel blocker, which could have contributed to this constellation of symptoms; both beta blocker and calcium channel blocker toxicity can lead to hypotension, cardiogenic shock, and bradycardia. Patient had been taking large doses of diltiazem and metoprolol, both in the setting of acute kidney injury which may have potentiated their respective effects secondary to decreased clearance. Intravenous calcium has been shown to reverse beta blocker and calcium channel blocker associated toxicities. In a paper by Henry et al, two cases of oral beta blocker and calcium channel blocker administration associated with profound hypotension and bradycardia were treated with intravenous calcium chloride with an immediate and pronounced improvement in hemodynamics. 11 In the case presented here, intravenous calcium chloride, albeit administered a few hours prior to the bradycardia and hypotension, had no effect on the hemodynamics. A study by Wagner and Salzer found an association between digoxin toxicity and calcium levels. 12 On isolated guinea‐pig papillary muscle as well as the atrium, increasing calcium concentrations were associated with increased digoxin toxicity. 12 Nevertheless, treatment of this condition necessitated initiation of vasopressors given the patient's significant hypotension and bradycardia. Dopamine was initiated given it's marked effect on blood pressure and heart rate at certain concentrations. The echocardiogram performed just prior to initiation of dopamine showed an ejection fraction of approximately 15%–20% which recovered with digibind, dopamine, and epinephrine indicating reversibility of this condition with pressor support, binding, and eventual removal of the offending agent.
One study on the development of stone hearts in pigs reported that the fixed contraction could be due to a calcium overload and/or sensitivity increase. 9 The change in troponin structure seems to alter the myofilament sensitivity to calcium. This change causes a contractile activation that would deplete energy in the form of ATP. Without ATP, myosin cannot unbind from the actin filaments, leaving myosin in a state of contraction. 13
Other studies report no significant relationship between calcium administration in digoxin toxicity and a stone heart. In a study by Levine and colleagues, 23 out of 159 patients with digoxin toxicity were given calcium, and mortality rates between groups were insignificant (22% vs. 20%, respectively, p‐value 0.78). 6 In another study using porcine models, digoxin toxicity was induced in both groups, and the animals were given either IV calcium chloride or IV saline at three different time intervals. Results showed no statistically significant differences in mortality rates between groups at any interval. 14
A 2022 paper by Peters et al attempted to address outcomes in patients who were treated for digoxin toxicity with or without anti‐digoxin Fab. 15 They identified 727 patients at a single center between 2000 and 2020 with signs and symptoms of digoxin toxicity defined by a primary diagnosis code of toxicity and/or anti‐digoxin Fab order and/or hospital admission or emergency department visit with elevated digoxin serum concentrations of greater than 2 ng/mL. 15 Mortality rate was found to be 12.7% inpatient and 42.7% at 1 year. Anti‐digoxin Fab was administered in 9% of those patients. 15 Those administered anti‐digoxin Fab were noted to have a greater burden of comorbidities, lower heart rates on presentation, worse renal function, and a higher serum potassium level. 15 In addition, these same patients had a numerically lower in‐patient mortality which was not significant (8.2% vs. 15.8%, p = 0.199), 30‐day all‐cause hospitalization (14.3% vs. 24.7%, p = 0.112) and comparable 6 and 12 month mortality and hospitalizations. 15
Given that our case revealed a potential link between intravenous calcium administration and the rare occurrence of a “stone heart” in the setting of digoxin toxicity, we must remain prudent. Despite studies suggesting a lack of association, this occurrence should always be considered in the differential diagnosis in the management of similar clinical scenarios.
LEARNING POINTS/TAKE HOME MESSAGES
Digoxin toxicity should remain in the differential for any unexplained hyperkalemia for patients on digoxin.
IV Calcium treatment should be limited to EKG changes secondary to hyperkalemia and avoided in cases of suspected digoxin toxicity.
Treatment for suspected digoxin toxicity with anti‐digoxin Fab should not be delayed in alleged cases of digoxin toxicity.
AUTHOR CONTRIBUTIONS
Guleid Hussein: Conceptualization; writing – original draft. Peter Krastev: Writing – original draft. Arvin Junn P. Mallari: Writing – original draft. Mohamad El Labban: Writing – review and editing. Sumeet Yadav: Writing – review and editing. Mohamed Hassan: Writing – review and editing. Mohamed Warsame: Writing – review and editing. Greta Zoesch: Writing – review and editing. John Trnka: Writing – review and editing. Shika M. Jain: Writing – review and editing. Nikhil Vojjala: Writing – review and editing. Salim Surani: Writing – review and editing. Syed Anjum Khan: Conceptualization.
FUNDING INFORMATION
The corresponding author is a faculty member of Texas A&M University. Per the Whiley/Hindawi and Texas A&M University agreement, the author does not have to pay the APC.
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflict to disclose.
ETHICS STATEMENT
Being a case report, no IRB approval is required.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Hussein G, Krastev P, Mallari AJP, et al. Stone heart syndrome: A curious case of digoxin toxicity and calcium infusion. Clin Case Rep. 2024;12:e9376. doi: 10.1002/ccr3.9376
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Hald M, Hønge J, Dall RP, Larsen SH. Two cases of "stone heart" with fatal outcomes. J Thorac Dis. 2018;10(1):E74‐E76. doi: 10.21037/jtd.2017.12.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. David MNV, Shetty M. Digoxin. StatPearls [Internet]. Treasure Island (FL). StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556025/ [Google Scholar]
- 3. Cummings ED, Swoboda HD. Digoxin Toxicity. StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470568/ [PubMed] [Google Scholar]
- 4. Piper HM, Meuter K, Schäfer C. Cellular mechanisms of ischemia‐reperfusion injury. Ann Thorac Surg. 2003;75(2):S644‐S648. doi: 10.1016/s0003-4975(02)04686-6 [DOI] [PubMed] [Google Scholar]
- 5. Simon LV, Hashmi MF, Farrell MW. Hyperkalemia. StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470284/ [PubMed] [Google Scholar]
- 6. Levine M, Nikkanen H, Pallin DJ. The effects of intravenous calcium in patients with digoxin toxicity. J Emerg Med. 2011;40(1):41‐46. doi: 10.1016/j.jemermed.2008.09.027 [DOI] [PubMed] [Google Scholar]
- 7. Digoxin: current clinical uses and management of toxicity. Br J Cardiol (2023). Retrieved August 27, 2024 from: https://bjcardio.co.uk/2023/06/digoxin‐current‐clinical‐uses‐and‐management‐of‐toxicity/ [Google Scholar]
- 8. Bania TC, Blaufeux B, Hughes S, Almond GL, Homel P. Calcium and digoxin vs. calcium alone for severe verapamil toxicity. Acad Emerg Med. 2000;7:1089‐1096. [DOI] [PubMed] [Google Scholar]
- 9. Levine MD, O'Connor AD. Digitalis (cardiac glycoside) poisoning. 2024. UpToDate. Retrieved March 26, 2024 from https://www.uptodate.com/contents/digitalis‐cardiac‐glycoside‐poisoning?search=chronic%20digoxin%20toxicity§ionRank=1&usage_type=default&anchor=H7&source=machineLearning&selectedTitle=1%7E150&display_rank=1#H28
- 10. Andrews P, Anseeuw K, Kotecha D, Lapostolle F, Thanacoody R. Diagnosis and practical management of digoxin toxicity: a narrative review and consensus. Eur J Emerg Med. 2023;30(6):395‐401. doi: 10.1097/MEJ.0000000000001065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henry M, Kay MM, Viccellio P. Cardiogenic shock associated with calcium‐channel and beta blockers: reversal with intravenous calcium chloride. Am J Emerg Med. 1985;3(4):334‐336. doi: 10.1016/0735-6757(85)90060-9 [DOI] [PubMed] [Google Scholar]
- 12. Wagner J, Salzer WW. Calcium‐dependent toxic effects of digoxin in isolated myocardial preparations. Arch Int Pharmacodyn Ther. 1976;223(1):4‐14. [PubMed] [Google Scholar]
- 13. Li M, Qin Z, Steen E, et al. Development and prevention of ischemic contracture ("stone heart") in the pig heart. Front Cardiovasc Med. 2023;10:1105257. doi: 10.3389/fcvm.2023.1105257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hack JB, Woody JH, Lewis DE, Brewer K, Meggs WJ. The effect of calcium chloride in treating hyperkalemia due to acute digoxin toxicity in a porcine model. J Toxicol Clin Toxicol. 2004;42(4):337‐342. doi: 10.1081/clt-120039538 [DOI] [PubMed] [Google Scholar]
- 15. Peters AE, Chiswell K, Hofmann P, Ambrosy A, Fudim M. Characteristics and outcomes of suspected digoxin toxicity and immune fab treatment over the past two decades—2000‐2020. Am J Cardiol. 2022;183:129‐136. doi: 10.1016/j.amjcard.2022.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


